Microreviews:
Cell Stress, Vol. 4, No. 2, pp. 44 - 47; doi: 10.15698/cst2020.02.213
Biomechanical stress provides a second hit in the establishment of BMP/TGFβ-related vascular disorders
1 Freie Universität Berlin, Institute for Chemistry and Biochemistry, 14195 Berlin, Germany.
Keywords: BMP, TGFβ, PAH, BMPR2, Integrin, LAP, fibrillin.
Abbreviations:
BMP – Bone Morphogenetic Protein;
BMPR2 – BMP Receptor Type II;
EC – endothelial cell;
ECM – extracellular matrix;
EndMT – endothelial-to-mesenchymal transition;
HHT – hereditary hemorrhagic telangiectasia;
HPAH – hereditary PAH;
iem – internal elastic membrane;
IPAH – idiopathic PAH;
LAP – latency associated peptide;
PAH – Pulmonary Arterial Hypertension.
Received originally: 10/01/2020 Accepted: 16/01/2020
Published: 20/01/2020
Correspondence:
Petra Knaus, Freie Universität Berlin, Institute for Chemistry and Biochemistry, 14195 Berlin, Germany; petra.knaus@fu-berlin.de
Conflict of interest statement: The authors declare no conflict of interest.
Please cite this article as: Christian Hiepen, Jerome Jatzlau and Petra Knaus (2020). Biomechanical stress provides a second hit in the establishment of BMP/TGFβ-related vascular disorders. Cell Stress 4(2): 44-47. doi: 10.15698/cst2020.02.213
Cardiovascular disorders are still the leading cause for mortality in the western world and challenge economies with steadily increasing healthcare costs. Understanding the precise molecular pathomechanisms behind and identifying players involved in the early onset of cardiovascular diseases remains crucial for the development of new therapeutic strategies. Taking advantage of CRISPR/Cas9 gene editing in human endothelial cells (ECs), we re-investigated the early molecular steps in a genetic vascular disorder termed pulmonary arterial hypertension (PAH) in our recent study (Hiepen C., Jatzlau J. et al.; PLOS Biol, 2019). Here, mutations in the Bone Morphogenetic Protein type II receptor (BMPR2) prime for the hereditary form (HPAH) with downregulated BMPR2 followed by a characteristic change in SMAD signaling, i.e. gain in both SMAD1/5 and SMAD2/3 responses. Remarkably these cells show increased susceptibility to signaling by TGFβ due to remodeling of the extracellular matrix (ECM) and increased biomechanics acting as a secondary stressor for ECs pathobiology. This clearly places BMPR2 not only as a BMP-signaling receptor, but also as a gatekeeper to protect ECs from excess TGFβ signaling.
BMPR2 MUTATIONS PRIME FOR PAH BUT REQUIRE A SECOND HIT
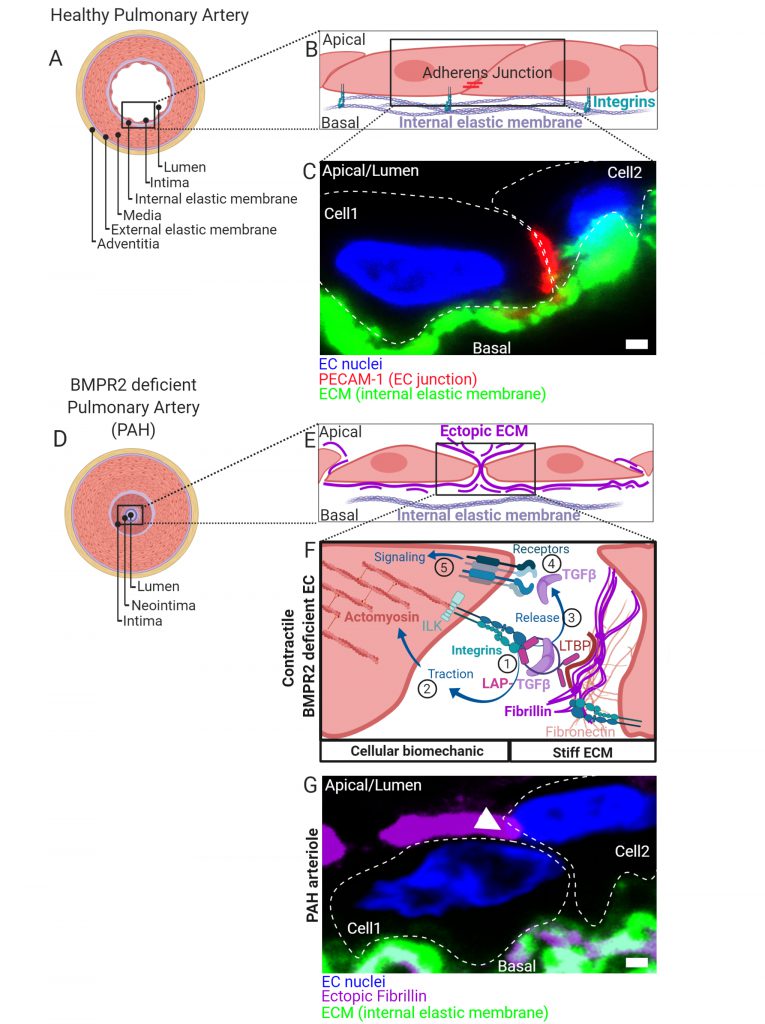
While BMPR2 mutations appear as one of the main genetic drivers for HPAH, low BMPR2 expression is also found in idiopathic cases (IPAH). Therefore, restoring BMPR2 expression became a wishful thinking in the design of PAH treatment with new attempts in current clinical pipelines. It is proposed that mutant BMPR2 is not sufficient to cause PAH and that an additional stressor is needed for disease onset following the “second-hit” concept known from cancer pathologies. A healthy pulmonary artery is characterized by a discrete tissue architecture with EC-derived intima, an internal elastic membrane (iem) composed of a defined mixture of ECM proteins, followed by multiple layers of smooth muscle cells (SMCs) separating the outer adventitia (Fig. 1A). EC attachment to the iem is facilitated at the basal side by integrin complexes engaging with the ECM, while junctional cell-to-cell contacts are established by intermolecular interaction of e.g. adherence junction proteins (Fig. 1B, C). Early pathomechanisms in PAH are media hypertrophy, intima thickening, neointima formation (Fig. 1D) and obstruction of small arterioles leading to increased blood pressure in the lung. This will consequently challenge the right heart ventricle. Amongst the secondary stressors proposed are imbalanced hormones, acute inflammation, hypoxia, protein misfolding with ER stress and epigenetic triggers. In search for secondary stressors that are conceptually different to the ones proposed, we speculated that the mechanical interaction of ECs with their extracellular environment could be of similar importance. We proposed that loss of BMPR2 drives cells towards a prestressed biomechanical state, allowing them to initiate a vicious feed-forward TGFβ auto-stimulation cycle.
–
BMPR2 DEFICIENCE FAVORS BIOMECHANICAL TGFβ RETRIEVAL FROM THE ECM
We could show, that BMPR2-deficient cells change their biomechanics via differential expression and ectopic depo-sition of ECM components such as fibrillin-1 and fibron-ectin (Fig. 1E). This gives rise to a mechanical micro-milieu permissive to harbor high amounts of the latent and biologically inactive form of TGFβ (latent TGFβ). Latent TGFβ is bound to a latency associated peptide (LAP) and tethered to fibrillin microfibrils (Fig. 1F). First, binding of cell-surface integrins to the RGD sequence within LAP of TGFβ (1) is followed by actomyosin-dependent traction forces (2) which mechanically activate and release mature TGFβ (3). We found that BMPR2-deficient cells express high levels of activated RDG binding β1-integrin and show increased Rho kinase-dependent contractility. By this mechanism, mature and biologically active TGFβ is released from its extracellular depot to thus bind to TGFβ-receptor complexes on the cell surface (4) and to induce cellular signaling in an auto-stimulatory manner (5) (Fig. 1F).
ENDMT AND JUNCTIONAL REORGANIZATION IN RESPONSE TO BIOMECHANICAL STRESS
Excessive TGFβ signaling in ECs induces trans-differentiation towards a mesenchymal-like phenotype, a process termed endothelial-to-mesenchymal transition (EndMT). Indeed, in BMPR2 deficient cells we found structural alterations such as increased actin stress-fiber formation and changes in stress fiber stiffness and subcellular localization, together with increased expression of mesenchymal markers. Interestingly, in the absence of BMPR2 we could localize both β1-integrin and integrin-linked kinase (ILK) at cell contact sites culminating in major changes in the junctional architecture highlighted by the loss of VE-Cadherin and PECAM-1. In fact, we found resolution of EC junctions accompanied by ectopic fibrillin deposits that atypically span through cell contact sites to project apically (Fig. 1G). When cultured under steady state conditions, BMPR2-deficient cells were mechanically capable for the retrieval of active TGFβ from its latent ECM depot. Ectopic fibrillin deposits and signs of EndMT were also found in the intima regions of human pulmonary arteries from PAH patients (Fig. 1G). Thus, it is possible that the mechanically driven auto-stimulation with TGFβ contributes to EndMT in PAH. Biomechanical driven TGFβ release and ectopic ECM production is a process known from scar formation and fibrosis and is primarily a feature of fibroblasts. Indeed, our findings confirm that EndMT of BMPR2-deficient ECs gives rise to a myo-fibrotic cell type, which is potently auto-stimulated by TGFβ. Mechanical TGFβ release and auto-stimulation seems to promote the disease onset at the EC layer in blood vessels. Thus, biomechanics acts as a bona fide secondary stressor in this process.
COMMONALITIES AND OUTLOOK ON BMP/TGFβ RELATED VASCULAR DISORDERES
Interestingly, vascular diseases such as hereditary hemorrhagic telangiectasia (HHT), Marfan syndrome and Loeys-Dietz-syndrome may be conceptually related. Even though clinically distinct, PAH, HHT, Marfan- and Loeys-Dietz- syndrome may be driven by a similarly occurring biomechanical stressor because they show dysregulation of the same molecular players found in BMPR2-deficient ECs (i.e. TGFβ, BMP9, endoglin, ALK1, Alk5, fibrillin and SMADs). While the molecules involved appear conserved, the biomechanics of each vascular bed in which clinical symptoms develop is very distinct ranging from aorta to small arterioles and capillaries. It would be therefore very important to identify commonalities between these vascular diseases and to develop translational approaches implementing experts in biophysics, biochemistry and medicine. Further action has to be taken to unveil if our findings have clinical relevance. It raises the question whether a direct targeting of cellular biomechanics i.e. “making cells more relaxed” should be considered when developing new therapeutic intervention strategies for PAH.
ACKNOWLEDGMENTS
P.K. and C.H. were funded by Morbus Osler Foundation/ Morbus Osler Selbsthilfe e.V. Germa-ny. Research on this topic was funded by the Deutsche Forschungsgemeinschaft DFG: SFB 958, FOR 2165, the Berlin Brandenburg School for Regenerative Therapies and the Einstein Center for Regenerative Therapies. The ethics committee of the federal state Berlin approved the methods used to analyze human PAH samples pro-vided to PK (EA2/082/19).
COPYRIGHT
© 2020

Biomechanical stress provides a second hit in the establishment of BMP/TGFβ-related vascular disorders by Hiepen et al. is licensed under a Creative Commons Attribution 4.0 International License.