Back to article: Mitochondrial stress management: a dynamic journey
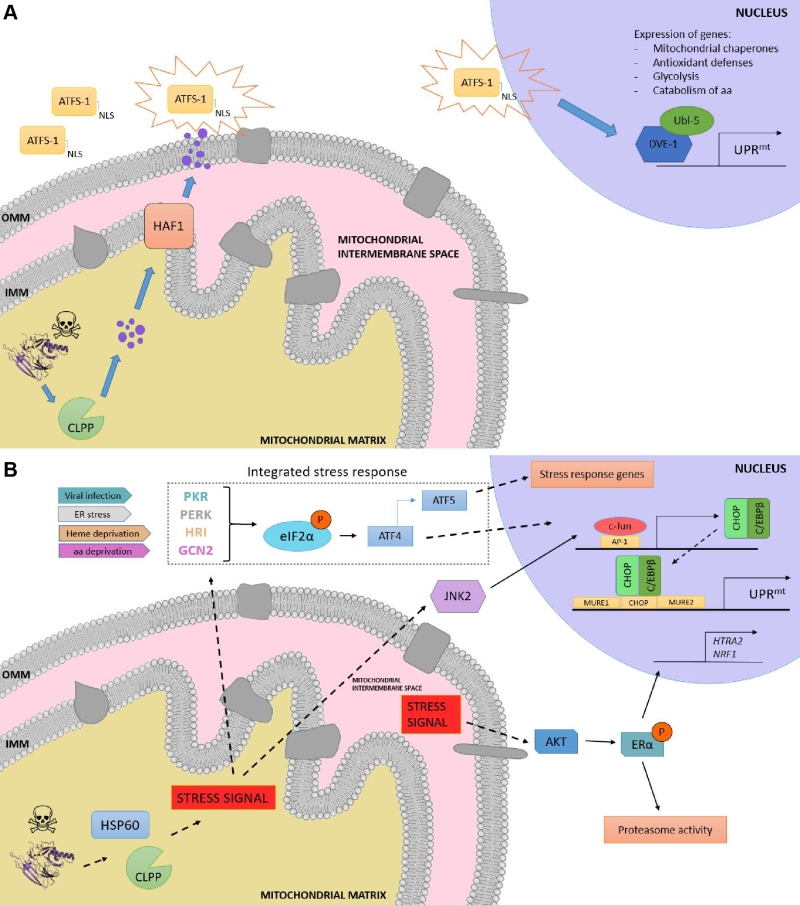
FIGURE 3: Overview of the UPRmt in worms and mammals. (A) UPRmt in C. elegans. When the capability of the mitochondria to process unfolded proteins is compromised, the mitochondrial unfolded protein response (UPRmt) is activated. Upon mitochondrial stress, the protease CLPP degrades the excess of unfolded proteins, and the resulting fragments are moved across the inner mitochondrial membrane (IMM) to the intermembrane space, where they diffuse through passive diffusion to the cytoplasm. ATSF-1 import inside mitochondria is prevented, and its activation upon the sensing of the degraded peptides shuttles ATFS-1 to the nucleus, granted by the nuclear localization sequence (NLS). (B) Retrograde signaling in mammals upon proteotoxic stress. The excess of unfolded proteins is sensed by quality control proteases and chaperones such as HSP60 and CLPP, and the stress signal is processed via two different pathways, the activation of the CHOP gene via the JNK pathway and the induction of the integrated stress response (ISR), respectively. On the one hand, JNK2 processes the stress signal from the mitochondrial matrix to activate the transcription factor c-Jun, which binds to AP-1 elements and induces the expression of CHOP and C/EBPβ. The CHOP-C/EBPβ complex then binds to the CHOP element, which is flanked by two mitochondrial unfolded protein response elements (MURE1 and MURE2), activating the transcription of UPRmt -responsive genes. On the other hand, viral infection, ER stress, heme deprivation and amino acid insufficiency activate PRK, PERK, HRI and GCN2, respectively, giving rise to the phosphorylation of eIF2α, the core of the ISR. This leads to the preferential translation of ISR-specific mRNAs, such as ATF4, the main effector of the ISR. ATF4 enhances the transcription of ATF5, leading consequently to the transcription of target genes. Moreover, a matrix-independent IMS- specific UPRmt has also been reported. Stress signals coming from the accumulation of unfolded proteins in the IMS leads to the AKT-mediated phosphorylation of ERα, resulting in the expression of HTRA2, NRF1, and an increase in the activity of the proteasome.