Back to article: Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase
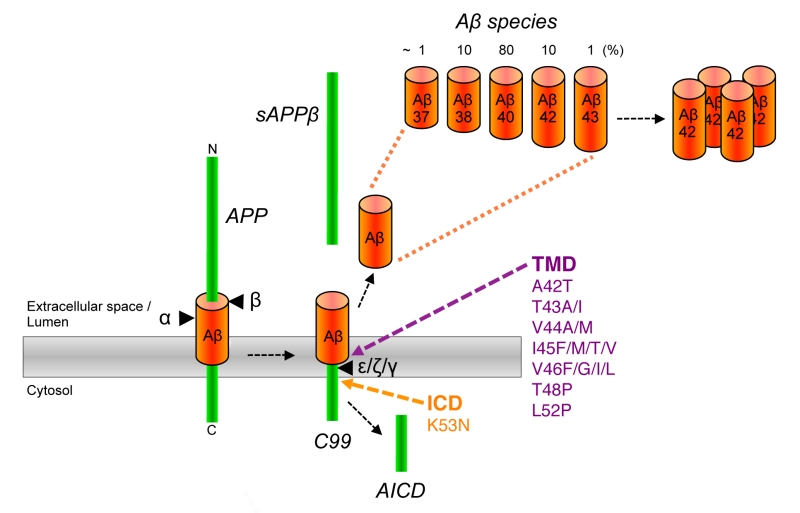
FIGURE 1: APP processing and generation of Aβ. APP is first cleaved by β-secretase (β) in its ectodomain close to the extracellular/luminal membrane border thereby generating a 99 amino acid C-terminal APP fragment (C99). Consecutive cleavages of C99 by γ-secretase at ε-, ζ-, and γ-sites releases the APP intracellular domain (AICD) into the cytosol and 37-43 amino acid Aβ species into the extracellular space or lumen of secretory pathway organelles. Longer Aβ forms such as in particular Aβ42 are highly aggregation prone and ultimately deposit as plaques in AD patient brains. An alternative cleavage of APP by α-secretase (α) in the Aβ domain prevents the formation of Aβ. Pathogenic APP FAD mutations that have been shown or are likely to cause relative increases in the generation of Aβ42 species are located in the γ-secretase cleavage region of the APP TMD and in the AICD.