Reviews:
Cell Stress, Vol. 6, No. 10, pp. 79 - 88; doi: 10.15698/cst2022.10.272
Pyroptosis in NLRP3 inflammasome-related atherosclerosis
1 School of Public Health, International Science and Technology Cooperation Base of Air Pollution and Health, Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou 310053, Zhejiang Province, China.
2 School of Basic Medical Science, Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou 310053, Zhejiang Province, China.
3 First Affiliated Hospital of Xinxiang Medical University, Weihui 453100, Henan Province, China.
4 Laboratory of Environmental Medicine and Developmental Toxicology, Guangdong Key Laboratory of Environmental Pollution and Health, School of Environment, Jinan University, Guangzhou 511443, Guangdong Province, China.
# These authors contributed equally to this work.
Keywords: NLRP3 inflammasome, caspase-1, GSDMD, IL-1β, pyroptosis, atherosclerosis, cardiovascular diseases.
Received originally: 27/06/2022 Received in revised form: 10/09/2022
Accepted: 12/09/2022
Published: 10/10/2022
Correspondence:
Xiang Zeng, School of Public Health, Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou 310053, Zhejiang Province, China; zengxiang3044@163.com
Conflict of interest statement: The authors declare that they have no conflict of interest.
Please cite this article as: Xiang Zeng, Dongling Liu, Xia Huo, Yue Wu, Cuiqing Liu, Qinghua Sun (2022). Pyroptosis in NLRP3 inflammasome-related atherosclerosis. Cell Stress 6(10): 79-88. doi: 10.15698/cst2022.10.272
Abstract
Pyroptosis is a proinflammatory form of programmed cell death in response to inflammation. It involves in the pathogenesis and outcomes of atherosclerosis characterized by NLRP3 inflammasome assembly, membrane pore formation, cell swelling, pro-inflammatory mediator and cytokine release. There are known pyroptosis molecular pathways including the caspase-1 depended canonical signaling pathway and the caspase-4/5/11 determined non-canonical signaling pathway. It is essential to explore the connection among NLRP3 inflammasome, pyroptosis and atherosclerosis, which may shed light on the potential therapeutic strategies that target pyroptosis in atherosclerotic treatment.
INTRODUCTION
Cardiovascular diseases (CVDs) are the leading causes of mortality and morbidity globally, which was estimated that 17.9 million individuals died due to CVDs in 2019, accounting for 32% of all global deaths [1]. Atherosclerosis is a cardiovascular progressive lesion occurred in coronary, cerebral, or peripheral vessels etc., resulting in serious health threat, heavy social and economic burdens [2][3][4]. It is characterized by the thickening of intima and the formation of plaque at sites with endothelial cell injury and chaotic laminar flow, and has been regarded as a metabolic inflammatory disorder [5][6][7].
–
Nucleotide-binding oligomerization domain-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome is an intracellular molecular platform consisting of NLRP3, ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and pro-caspase-1, and may be activated by a diverse range of stimuli such as pathogens, irritants, apoptotic and pyroptotic cells [8][9][10]. Additionally, NLRP3 inflammasome plays a crucial role in inflammatory response to defend external stimuli. Potassium (K+) eflux, calcium (Ca2+) waves, lysosome disruption, mitochondrial damage are the main activators for NLRP3 inflammasome activation in the body [11]. Activation of NLRP3 inflammasome has been documented in the pathogenesis of atherosclerosis, and elevated NLRP3 inflammasome levels were recorded in atherosclerotic patients [12][13][14][15]. However, little is known about the molecular mechanism underlying how NLRP3 inflammasome impacts atherosclerosis.
–
Recently, emerging studies report that pyroptosis is associated with both NLRP3 inflammasome and atherosclerosis [7][16][17][18][19]. Some studies demonstrate that pyroptosis is a caspase-dependent pro-inflammatory form of programmed cell death, which is characterized by activation of NLRP3 pathways, pore formation of cell membrane, and maturation and release of pro-inflammatory mediators such as interleukin (IL)-1β, IL-18, and pore-forming protein gasdermin D (GSDMD) [20][21][22]. Therefore, this study explores the mediator’s role of pyroptosis between NLRP3 inflammasome and atherosclerosis, which may shed light on the molecular mechanism and therapeutic potential in the origination and progression of atherosclerosis.
NLRP3 INFLAMMASOME
Components and assembly of NLRP3 inflammasome
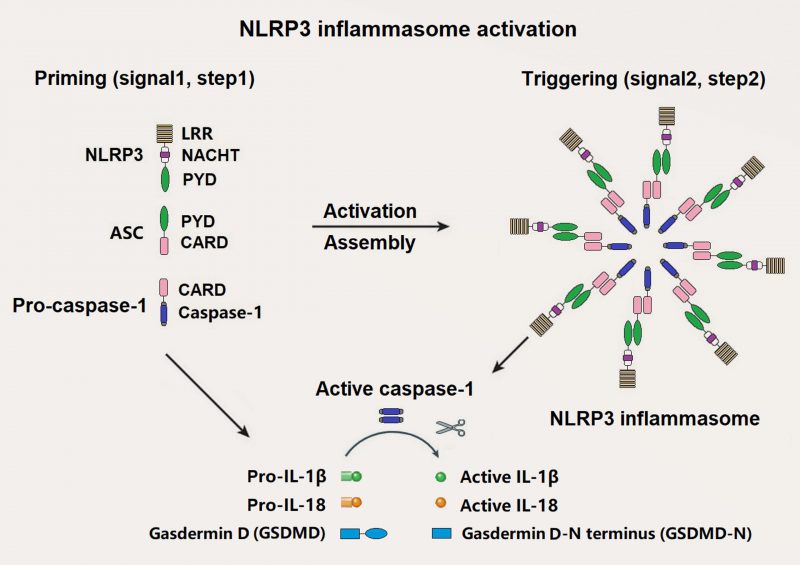
NLRP3 inflammasome is one of the best known inflammasomes, which is consisted of NLRP3 sensor, ASC adaptor, and caspase-1 effector [10]. NLRP3 consists of three domains: An N-terminal effector pyrin domain (PYD), a central nucleotide-binding and oligomerization (NACHT) domain and a C-terminal leucine-rich repeats (LRRs) domain. ASC is made up of an N-terminal PYD and a C-terminal caspase recruitment domain (CARD). Pro-caspase-1 is composed of a CARD and caspase domains (Figure 1). Activated NLRP3 recruits ASC and pro-caspase-1 through a homotypic interaction of PYD-PYD and CARD-CARD, respectively.
–
–
Activation of NLRP3 inflammasome
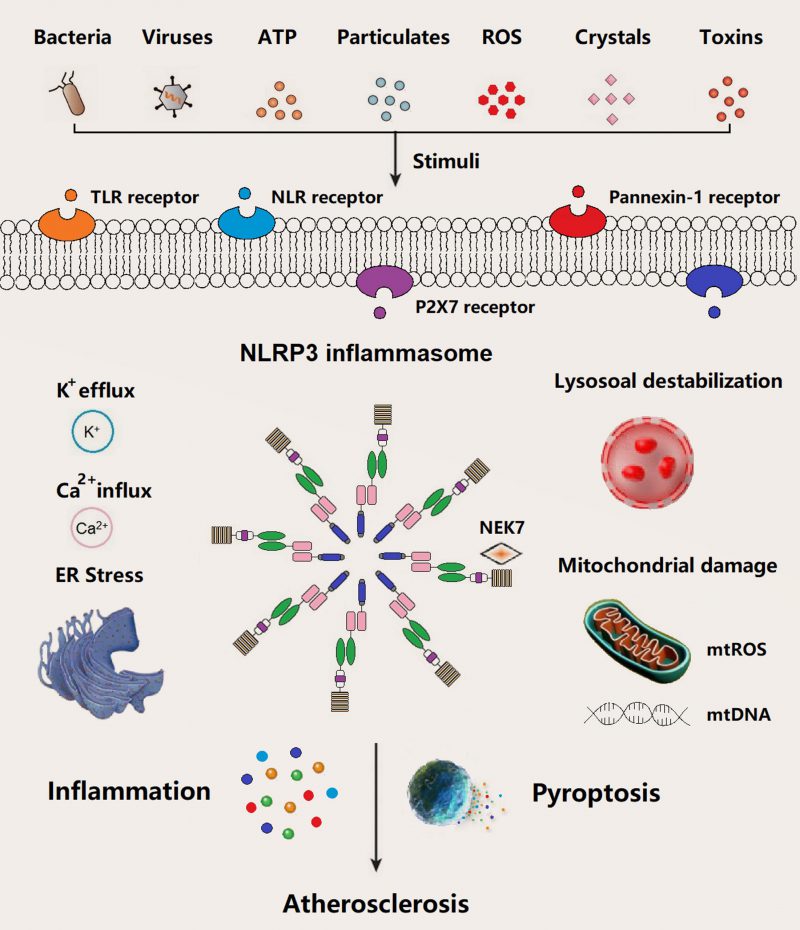
In general, the basal expression level of NLRP3 is not enough to activate NLRP3 inflammasome until adequate ASC and pro-caspase-1 are readily in the activated state. NLRP3 inflammasome starts the assembly process in response to infection or sterile inflammation induced by a variety of stimuli such as bacteria, virus, adenosine triphosphate (ATP), particulates, reactive oxygen species (ROS), crystals, and pore-forming toxins (Figure 2). Extracellular ATP, particulate matter, crystals, and pore-forming toxins are common up-regulators of NLRP3 inflammasome activation [23]. Above events in relation to NLRP3 inflammasome activation have been documented, mainly including potassium (K+) eflux, calcium (Ca2+) influx, lysosome destabilization and rupture, mtROS and mtDNA damage, and endoplasmic reticulum (ER) stress. Several intracellular pathways, i.e., nuclear factor κB (NF-κB), mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinases (JNK) signaling involved in the assembly process of NLRP3 inflammasome, were activated by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).
–
NLRP3 INFLAMMASOME IN ATHEROSCLEROSIS
The main characteristics of atherosclerosis are lipid accumulation, inflammatory cell infiltration, endothelial dysfunction, as well as proliferation of collagen and smooth muscle cells in endarterium [24]. Although dyslipidemia is the leading cause for atherosclerosis, inflammation is also a primary driver and modulator in the occurrence and progression of atherosclerosis. As the best well-known and dominant inflammasomes, NLRP3 inflammasome is involved in the formation and development of inflammation and its related diseases [10]. Several studies have been sprung up in exploring NLRP3 inflammasome in relation to atherosclerosis [25][26][27][28][29][30][31]. However, the results of NLRP3 inflammasome on atherosclerosis are inconsistent and its molecular mechanism is still uncertain.
–
IL-1β is one of the well-known inflammatory effector molecules during period of NLRP3 inflammasome activation, and also plays a pro-atherogenic role in the progression of atherosclerosis [32]. Therefore, IL-1β has been considered as a key mediator of NLRP3 inflammasome and atherosclerosis. It upregulates the secretion of adhesion molecules in both endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), and accelerates the aggregation of monocytes or macrophages to vascular wall [33][34]. Furthermore, IL-1β could also promote ECs and VSMCs to assemble into the intima and trigger the release of inflammatory cytokines and chemokines to further aggravate inflammation in macrophages [35]. In addition, L-18 share similar function with IL-1β, which tends to be considered as a proatherogenic cytokine in atherosclerosis [36][37][38]. It is worth noting that IL-1β mediates the activation of NLRP3 inflammasome and the pathogenesis of atherosclerosis.
–
Deficiency of NLRP3, ASC, and IL-1β in bone marrow significantly decreased atherosclerotic lesion formation [39][40][41]. In other words, the above-mentioned bioactive compounds tremendously contribute to development of atherogenesis [35][42][43]. Utilization of gene silencing and specific inhibitors of NLRP3 also demonstrated that there was an atherogenic role of NLRP3 inflammasome [44][45]. Additionally, a reduction in atherosclerotic lesion was found in both low-density lipoprotein receptor (LDLR)–/– mice and apolipoprotein E (ApoE)−/− mice with deficiency in caspase-1/11 [46][47][48]. However, a few studies reported that there was no significant alteration in plaque size or stability of atherosclerosis among ApoE−/− mice no matter whether deficiency of NLRP3, ASC, caspase-1 or not [49], which is inconsistent with the results of the above-mentioned studies. Dissimilar research background and conditions such as sex, atherogenic diet, and hyperlipidemia level may contribute to these conflicting results. For example, insufficiency NLRP3 alleviated atherogenesis in LDLR−/− female mice [40]. Notedly, NLRP3 inflammasome components have been upregulated in human atherosclerosis [14]. Moreover, NLRP3 protein level in peripheral leukocyte were linked with the severity of coronary atherosclerotic patients with acute coronary syndrome [50]. Taken together, NLRP3 inflammasome plays a key role in the development of atherosclerosis.
–
The underlying molecular mechanisms of NLRP3 inflammasome assemble in atherogenesis have been documented in recent years, such as K+ efflux (reduction of intracellular K+), Ca2+ influx (increase in intracellular Ca2+), cathepsin leakage by lysosomal destabilization, ER stress, and mtROS/mtDNA (Figure 2) [51][52][53]. Cholesterol crystals (CCs) are one of the most potential stimuli/activators of NLRP3 inflammasome, which is present in atherosclerotic plaques of all stages. The formation of CCs was induced by incorporating oxidized low-density lipoprotein (Ox-LDL) and CD36 scavenger receptor in macrophages, which can activate NLRP3 inflammasome [13][39][54]. Similar to other particle activators like particulate matter, pore-forming toxins, silica, and asbestos of NLRP3 inflammasome, CCs are mainly engulfed/phagocytosed by macrophages and subsequently accumulated in lysosomes. Inadequate digestion of CCs in lysosome may induce lysosomal membrane damage and subsequent leakage of cathepsins into cytoplasm, ultimately result in NLRP3 inflammasome activation [55][56][57]. Meanwhile, along with other particle substances, e.g., nigericin (a bacterial pore-forming toxin and K+ ionophore) and extracellular ATP (decrease of intracellular ATP), CCs activate NLRP3 inflammasome via K+ efflux pathway accompanied by purinergic P2X7 receptor (Figure 2) [58]. Additionally, NLRP3 inflammasome assemble can be triggered by calcium phosphate crystals, uric acid crystals, or fatty acid crystals [59][60][61][62]. Extracellular ATP derived from impaired or dead cells make up the necrotic core of atherosclerotic plaque, which triggers the NLRP3 inflammasome through P2X7/K+ efflux pathway. P2X7–/–/LDLR–/– mice or P2X7-short interference (si) RNA in ApoE–/– mice demonstrated a decline in atherosclerotic lesions [63][64][65]. Hypoxia, a driving factor of atherosclerosis, arose in atherosclerotic plaques, which can induce and enlarge NLRP3 inflammasome activation in macrophage [66][67][68]. Moreover, mtROS/mtDNA can also trigger NLRP3 inflammasome in atherosclerosis [69][70][71]. For example, decreased 8-oxoguanine glycosylase (a DNA glycosylase of eliminating oxidized DNA) in plaque macrophages led to elevated cytosolic oxidized mtDNA and subsequent NLRP3 inflammasome assembly [70]. Notably, NLRP3 inflammasome activation in atherogenesis occurs in a variety of cells such as macrophages, neutrophils, endothelial cells. Several other stimuli such as bacterial, viruses, particle matter, and ROS have been identified to be involved in the priming and triggering NLRP3 inflammasome in the process of atherosclerosis (Figure 2).
PYROPTOSIS IN ATHEROSCLEROSIS
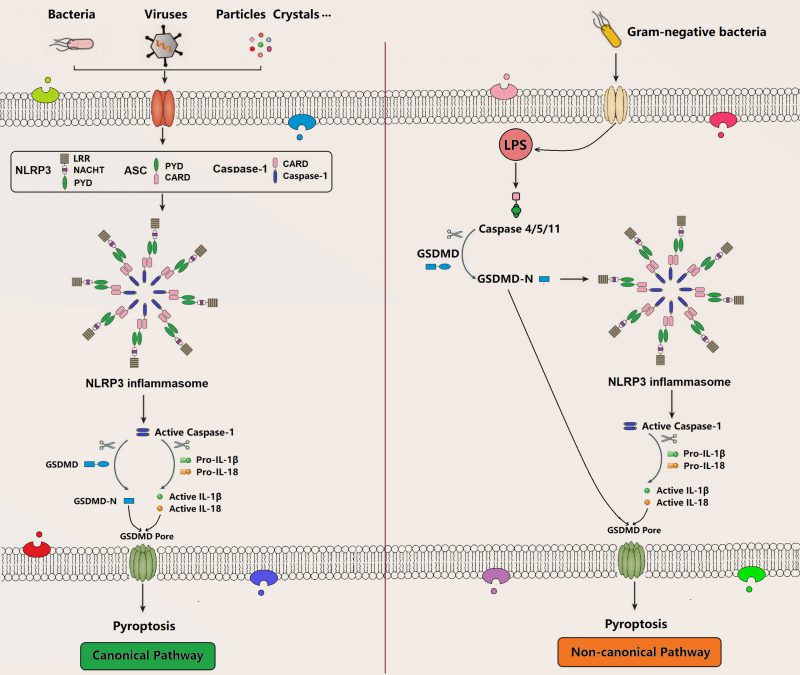
Pyroptosis is a recently identified form of programmed cell death, which is characterized by membrane pore formation (bubbling), cell swelling, and cell lysis. Morphologically, it is most likely to be a combination of apoptosis and necrosis. Pyroptosis can be activated by various PAMPs and DAMPs stimuli such as intracellular lipopolysaccharides (LPS), extracellular ATP, cytosolic DNA, bacterial flagella, and particle matters, which is accompanied by inflammasome assembly in terms of caspase-1 (canonical inflammasome pathway) or caspase-4/5/11 (noncanonical inflammasome pathway) that subsequently releases cell contents, secretes proinflammatory cytokines, and induces pyroptosis through the formation of membrane GSDMD pore (Figure 3) [72][73][74]. GSDMD is a member of the gasdermin (GSDM) family consisting of a gasdermin-N domain and a unique binding inhibitory domain. Upon activation, it can induce perforation and pyroptosis in mammalian cells. As a substrate of caspase-1, caspase-4, and caspase-5, caspase-11, GSDMD is also an important executor for pyroptosis mediating the pore-forming activity of membrane and the activation signaling of NLRP3 inflammasome [75][76][77][78][79]. There are several molecular pathways in pyroptosis, such as canonical and non-canonical pyroptosis signaling pathway [80].
–
–
The pathogenesis of atherosclerosis is characterized by recruitment of monocytes and lymphocytes, dysfunction of ECs, formation of foam cells (FCs), proliferation of smooth muscle cells (SMCs), secretion of proinflammatory cytokines, accumulation and oxidation of LDL, adherence of platelets, and death of abundant cells [7][81]. Previous studies have confirmed that atherosclerosis is mainly attributed to inflammation and the products of pyroptosis such as IL-1β, IL-18, IL-1α, ATP, and GSDMD-N, suggesting crucial role of pyroptosis in the pathogenesis of atherosclerosis [21][82][83]. Pyroptosis is closely in relation to the progress of atherosclerosis [7][16][84]; Additionally, extensive expression of NLRP3 was observed in ECs, macrophages, and SMCs [85]. Therefore, pyroptosis in ECs, macrophage, and SMCs is of a particularly noteworthy concern.
–
The ECs, locating and lining on the inner surface of vessel walls, are firstly exposure to, and highly susceptible to metabolite-related endogenous danger signals, which plays a critical role in maintaining the fluidity and thromboresistance of blood and regulating the permeability of vascular wall [86]. Previous studies reported that the impairment, loss of intima integrity, or dysfunction of ECs can initiate atherosclerosis [87][88][89]. The dysfunction of ECs is characterized by increased proinflammatory cytokines, elevated reactive oxidative species, chaotic vascular tone, and universal pyroptosis [7]. The activated ECs at the sites of inceptive atherosclerosis up-regulate the expression of P-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which are critical for attracting inflammatory cells, such as monocytes, for trans-endothelial recruitment [90]. Several cytokines and chemokines, such as IL-1α, ICAM-1, VCAM-1 and E-selectin, in ApoE-/-/caspase-1-/- mice were lower than those in ApoE-/- mice [91]. Additionally, pyroptotic ECs contribute to endothelial dysfunction in terms of elevating the permeability of endothelial monolayer and increasing the migration and deposition of lipids, monocytes, and SMCs into the intima accompanied by inflammatory cytokine release, and canonical pathway of NLRP3 is the main mechanism of ECs pyroptosis in atherosclerosis [16].
–
Foam cells, derived from macrophages, are the main cells engaged in the lesions of atherosclerosis and play a vital role in plaque instability [92]. Monocytes are the most numerous leukocytes in the plaques, which can differentiate into macrophages once they are recruited into arterial intima [21]. The death of macrophages can mediate the development of atherosclerotic lesions, which is manifested by promoting necrotic core formation, increasing plaque vulnerability, and accelerating thrombosis. It is worth pointing out that pyroptosis has been documented to contribute to a substantial proportion of macrophages death in atherosclerotic plaques [93]. Additionally, pyroptosis in atherosclerotic plaques induces inflammation, which leads to the migration of macrophage and SMCs, and the formation of foam cells. Previous studies reported that Ox-LDL and cholesterol crystals could trigger NLRP3 inflammasome and release bioactive IL-1β and IL-18 in macrophages [40]. Moreover, the activation of AIM2 inflammasome and subsequent formation of necrotic cores in macrophage pyroptosis can exacerbate atherosclerosis [94]. Taken together, these studies suggest that NLRP3-dependent pyroptosis in macrophages and foam cells may promote the formation of necrotic core formation and the instability of plaque, and further contribute to the progression of atherosclerosis.
–
Atherosclerotic plaque rupture is a common and important cause of thrombosis, which could further lead to myocardial infarction [95]. SMCs can produce extracellular matrix to form a fibrous cap through migrating from media layer to intima layer [96]. They are the main cellular components of atherosclerotic lesions, and play a pivotal role in the development and progression of vascular disorders [97]. The stability of atherosclerotic plaque is mainly depended on the thickness of the fibrous cap that is decreased in a state of inflammation [96]. The damage, dysfunction, or death of SMCs and subsequent collapse of extracellular matrix and collagen will thin the fibrous cap, which ultimately results in plaque rupture and complications related to it [98][99]. It has been reported that pyroptosis in SMCs promotes inflammation, which wrecks the fibrous cap [100]. The impairment of fibrous cap aggravates the instability and vulnerability of atherosclerotic plaque and increases the incidence of cardiovascular events [21]. Ox-LDL is a predominant component in atherosclerosis, which can lead to SMCs pyroptosis by activating NLRP3 inflammasome [16]. For example, VX-765, a specific inhibitor of caspase-1, inhibits the pyroptosis of SMCs [101]. Additionally, Ox-LDL induces caspase-1-mediated pyroptosis in SMCs [102]. Taken together, Ox-LDL may accelerate atherosclerotic plaque rupture by triggering SMCs pyroptosis, which may increase the instability of plaques and degrade fibrous cap through the pathway of NLRP3 inflammasome activation.
RELATION BETWEEN NLRP3 INFLAMMASOME AND PYROPTOSIS
Up-to-date, pyroptosis is a novelty pattern of GSDM-induced pro-inflammatory cell death characterized by the release of a large number of pro-inflammatory factors such as IL-1β and IL-18 in a variety of diseases. Interesting, IL-1β and IL-18 are the typical downstream bioactive products of NLRP3 inflammasome activation based on results of previous studies. A hypothesis was arisen that NLRP3 inflammasome may closely link with pyroptosis. In fact, a plenty of research results confirm the hypothesis in term of coexistence between activation of NLRP3 inflammasome and occurrence of pyroptosis in organism [103][104][105][106][107][108][109]. Many in-depth studies have found the molecular mechanism of NLRP3 inflammasome in relation to pyroptosis. Briefly, external or internal stimuli, such as microorganism, particulates, and ATP, can trigger NLRP3 inflammasome assembly and subsequently mature pro-caspase-1, which ultimately secret IL-1β and IL-18, and induce GSDMD-mediated pyroptosis. Specifically, NLRP3 inflammasome assembly promote pro-caspase-1 activation into active caspase-1, which accelerate pro-inflammatory factor release and GSDMD pore formation in cell membrane, and further induce pyroptosis [103]. NLRP3 inhibitors can alleviate or reverse the activation of NLRP3 inflammasome-mediated pyroptosis [104][105][106][107][108][109]. Blocking NLRP3 and pyroptosis is a new and effective strategy to inhibit inflammation and its related diseases, which may provide a unique perspective to deeply understand the relationship between NLRP3 inflammasome and pyroptosis [110].
MEDIATION AND THERAPEUTIC TARGETS OF PYROPTOSIS IN NLRP3 INFLAMMASOME AND ATHEROSCLEROSIS
The known signaling pathways of pyroptosis on atherosclerosis are nuclear factors such as NF-κB, AMPK, MAPK, SIRT, as well as miRNA, which may shed light on therapeutic targets for the treatment of atherosclerosis [111]. The vital role of pyroptosis in the pathogenesis of atherosclerosis has generated a few specific inhibitors or agents that target bioactive substances such as NLRP3, caspase-1, caspase-4/5/11, GSDMD, and other candidates in relation to pyroptosis pathway [7][81][84]. Pyroptosis-related atherosclerosis pathway covers the same targeted substances mentioned above, which may suggest that targeting NLRP3 inflammasome may be a therapeutic strategy to treat atherosclerosis. Previous studies have reported that NLRP3 inflammasome inhibitors such as adiponectin, allicin, angiotensin, artemisinin, Bay 11-7082, BOT-4-one, BRC36, CY-09, INF4E, MCC950, OLT1177, and oridonin, etc [11][84][112]. The known molecular mechanisms of these inhibitors of NLRP3 inflammasome include the impairment of ATPase activity, prevention of NLRP3 oligomerization, interference of ASC polymerization, obstruction of P2X7 channel, destabilization of the lysosome, or influence ATP/dATP binding in the central NACHT domain [88]. Additionally, caspase-1 inhibitors such as VX-765, Ac-YVAD-cmk, Ac-WEHD-CHO, and Pralnacasan can curb pyroptosis. GSDMD is an imperative pyroptosis executor and the cornerstone of transmembrane channels; the known GSDMD inhibitors, such as necrosulfonamide (NSA), Bay 11-7082 and disulfiram, can also block GSDMD membrane pore formation. Particularly, some pyroptosis associated non-coding RNAs such as miR-223, miR-30–5p, and lncR-MALAT1 have been documented to treat atherosclerosis [96]. General risk factors for atherosclerosis are Ox-LDL, acrolein, and low shear stress, etc. Therefore, drugs or agents that target these NLRP3 inflammasome-related substances could be promising or preventing pyroptosis-related diseases such as atherosclerosis.
CONCLUSION
As a proinflammatory form, pyroptosis plays a crucial role in the pathogenesis and complications of atherosclerosis that mainly targets ECs, macrophages, and SMCs. Pyroptosis in atherosclerotic lesions mainly depends on the NLRP3 inflammasome activation, and the signaling pathways involved provide some potential targets for novel therapeutic interventions in atherosclerosis. There still lacks in vivo studies and clinical trials that could provide a solid foundation for developing pyroptosis-inducing drugs. Future studies should concentrate on the molecular mechanisms of NLRP3-mediated pyroptosis in atherosclerosis and other pyroptosis-related chronic diseases.
REFERENCES
- WHO (2021). Cardiovascular diseases. World Health Organization. Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 [Accessed 11.06.2021]
- Xu H, Jiang J, Chen W, Li W, Chen Z (2019). Vascular Macrophages in Atherosclerosis. J Immunol Res 4354786. 10.1155/2019/4354786
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S (2016). Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res 118(4): 535-546. 10.1161/CIRCRESAHA.115.307611
- Lechner K, von Schacky C, McKenzie AL, Worm N, Nixdorff U, Lechner B, Krankel N, Halle M, Krauss RM, Scherr J (2020). Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol 27(4): 394-406. 10.1177/2047487319869400
- Liberale L, Montecucco F, Schwarz L, Luscher TF, Camici GG (2021). Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res 117(2): 411-422. 10.1093/cvr/cvaa211
- Back M, Yurdagul AJ, Tabas I, Oorni K, Kovanen PT (2019). Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 16(7): 389-406. 10.1038/s41569-019-0169-2
- He B, Nie Q, Wang F, Han Y, Yang B, Sun M, Fan X, Ye Z, Liu P, Wen J (2021). Role of pyroptosis in atherosclerosis and its therapeutic implications. J Cell Physiol 236(10): 7159-7175. 10.1002/jcp.30366
- Takahashi M (2021). NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc Res 118(2): 372-385. 10.1093/cvr/cvab010
- Mangan M, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018). Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17(8): 588-606. 10.1038/nrd.2018.97
- Liu D, Zeng X, Li X, Mehta JL, Wang X (2018). Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol 113(1): 5. 10.1007/s00395-017-0663-9
- Liu D, Zeng X, Li X, Cui C, Hou R, Guo Z, Mehta JL, Wang X (2020). Advances in the molecular mechanisms of NLRP3 inflammasome activators and inacativators. Biochem Pharmacol 175: 113863. 10.1016/j.bcp.2020.113863
- Grebe A, Hoss F, Latz E (2018). NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res 122(12): 1722-1740. 10.1161/CIRCRESAHA.118.311362
- Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H (2018). NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J Cell Physiol 233(3): 2116-2132. 10.1002/jcp.25930
- Paramel VG, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sorensen K, Skjelland M, Espevik T, Aukrust P, Lengquist M, Hedin U, Jansson JH, Fransen K, Hansson GK, Eriksson P, Sirsjo A (2016). NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J Am Heart Assoc 5(5): e003031. 10.1161/JAHA.115.003031
- Parsamanesh N, Moossavi M, Bahrami A, Fereidouni M, Barreto G, Sahebkar A (2019). NLRP3 inflammasome as a treatment target in atherosclerosis: A focus on statin therapy. Int Immunopharmacol 73: 146-155. 10.1016/j.intimp.2019.05.006
- Qian Z, Zhao Y, Wan C, Deng Y, Zhuang Y, Xu Y, Zhu Y, Lu S, Bao Z (2021). Pyroptosis in the Initiation and Progression of Atherosclerosis. Front Pharmacol 12: 652963. 10.3389/fphar.2021.652963
- Yu J, Cui X, Zhang X, Cheng M, Cui X (2021). Advances in the Occurrence of Pyroptosis: A Novel Role in Atherosclerosis. Curr Pharm Biotechnol 22(12): 1548-1558. 10.2174/1389201022666201231115034
- Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, Sun X, Li H, Zhang X, Yin S, Tan H (2020). NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol 34: 101523. 10.1016/j.redox.2020.101523
- Xue Y, Enosi TD, Tan WH, Kay C, Man SM (2019). Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol 40(11): 1035-1052. 10.1016/j.it.2019.09.005
- Zeng Z, Li G, Wu S, Wang Z (2019). Role of pyroptosis in cardiovascular disease. Cell Prolif 52(2): e12563. 10.1111/cpr.12563
- Xu YJ, Zheng L, Hu YW, Wang Q (2018). Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta 476: 28-37. 10.1016/j.cca.2017.11.005
- Yu ZW, Zhang J, Li X, Wang Y, Fu YH, Gao XY (2020). A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci 240: 117138. 10.1016/j.lfs.2019.117138
- Pelegrin P (2021). P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem Pharmacol 187: 114385. 10.1016/j.bcp.2020.114385
- Malekmohammad K, Bezsonov EE, Rafieian-Kopaei M (2021). Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front Cardiovasc Med 8: 707529. 10.3389/fcvm.2021.707529
- Karasawa T, Takahashi M (2017). Role of NLRP3 Inflammasomes in Atherosclerosis. J Atheroscler Thromb 24(5): 443-451. 10.5551/jat.RV17001
- Baldrighi M, Mallat Z, Li X (2017). NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 267: 127-138. 10.1016/j.atherosclerosis.2017.10.027
- Liu Y, Li C, Yin H, Zhang X, Li Y (2020). NLRP3 Inflammasome: A Potential Alternative Therapy Target for Atherosclerosis. Evid Based Complement Alternat Med 2020: 1561342. 10.1155/2020/1561342
- Chen X, Guo X, Ge Q, Zhao Y, Mu H, Zhang J (2019). ER Stress Activates the NLRP3 Inflammasome: A Novel Mechanism of Atherosclerosis. Oxid Med Cell Longev 2019: 3462530. 10.1155/2019/3462530
- Sharma BR, Kanneganti TD (2021). NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol 22(5): 550-559. 10.1038/s41590-021-00886-5
- Sharma A, Choi J, Stefanovic N, Al-Sharea A, Simpson DS, Mukhamedova N, Jandeleit-Dahm K, Murphy AJ, Sviridov D, Vince JE, Ritchie RH, de Haan JB (2021). Specific NLRP3 Inhibition Protects Against Diabetes-Associated Atherosclerosis. Diabetes 70(3): 772-787. 10.2337/db20-0357
- Rajamaki K, Mayranpaa MI, Risco A, Tuimala J, Nurmi K, Cuenda A, Eklund KK, Oorni K, Kovanen PT (2016). p38delta MAPK: A Novel Regulator of NLRP3 Inflammasome Activation With Increased Expression in Coronary Atherogenesis. Arterioscler Thromb Vasc Biol 36(9): 1937-1946. 10.1161/ATVBAHA.115.307312
- Takahashi M (2021). NLRP3 Inflammasome as a Common Denominator of Atherosclerosis and Abdominal Aortic Aneurysm. Circ J 85(12):2129-2136. 10.1253/circj.CJ-21-0258
- Kihara T, Toriuchi K, Aoki H, Kakita H, Yamada Y, Aoyama M (2021). Interleukin-1beta enhances cell adhesion in human endothelial cells via microRNA-1914-5p suppression. Biochem Biophys Rep 27: 101046. 10.1016/j.bbrep.2021.101046
- Guo J, Zhang H, Xia J, Hou J, Wang Y, Yang T, Wang S, Zhang X, Chen X, Wu X (2018). Interleukin-1beta induces intercellular adhesion molecule-1 expression, thus enhancing the adhesion between mesenchymal stem cells and endothelial progenitor cells via the p38 MAPK signaling pathway. Int J Mol Med 41(4): 1976-1982. 10.3892/ijmm.2018.3424
- Libby P (2017). Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol 70(18): 2278-2289. 10.1016/j.jacc.2017.09.028
- Yasuda K, Nakanishi K, Tsutsui H (2019). Interleukin-18 in Health and Disease. Int J Mol Sci 20(3). 10.3390/ijms20030649
- Kumari P, Russo AJ, Shivcharan S, Rathinam VA (2020). AIM2 in health and disease: Inflammasome and beyond. Immunol Rev 297(1): 83-95. 10.1111/imr.12903
- Watanabe D, Guo Y, Kamada N (2021). Interaction between the inflammasome and commensal microorganisms in gastrointestinal health and disease. EMBO Mol Med 13(12): e13452. 10.15252/emmm.202013452
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464(7293): 1357-61. 10.1038/nature08938
- Chen S, Markman JL, Shimada K, Crother TR, Lane M, Abolhesn A, Shah PK, Arditi M (2020). Sex-Specific Effects of the Nlrp3 Inflammasome on Atherogenesis in LDL Receptor-Deficient Mice. JACC Basic Transl Sci 5(6): 582-598. 10.1016/j.jacbts.2020.03.016
- Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y, Fan D, Tan H (2017). Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Invest 97(8): 922-934. 10.1038/labinvest.2017.30
- Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK (2012). Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest 122(1): 70-79. 10.1172/JCI43713
- Mai W, Liao Y (2020). Targeting IL-1beta in the Treatment of Atherosclerosis. Front Immunol 11(589654. 10.3389/fimmu.2020.589654
- Zheng F, Xing S, Gong Z, Mu W, Xing Q (2014). Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm 2014(507208. 10.1155/2014/507208
- van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, Foks AC, Bot I, Kuiper J (2017). NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler Thromb Vasc Biol 37(8): 1457-1461. 10.1161/ATVBAHA.117.309575
- Gage J, Hasu M, Thabet M, Whitman SC (2012). Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol 28(2): 222-229. 10.1016/j.cjca.2011.10.013
- Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M (2012). Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun 425(2): 162-168. 10.1016/j.bbrc.2012.07.058
- Hendrikx T, Jeurissen ML, van Gorp PJ, Gijbels MJ, Walenbergh SM, Houben T, van Gorp R, Pottgens CC, Stienstra R, Netea MG, Hofker MH, Donners MM, Shiri-Sverdlov R (2015). Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr(-/-) mice. FEBS J 282(12): 2327-2338. 10.1111/febs.13279
- Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J (2011). Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2(3): e137. 10.1038/cddis.2011.18
- Afrasyab A, Qu P, Zhao Y, Peng K, Wang H, Lou D, Niu N, Yuan D (2016). Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessels 31(8): 1218-1229. 10.1007/s00380-015-0723-8
- Jin Y, Fu J (2019). Novel Insights Into the NLRP 3 Inflammasome in Atherosclerosis. J Am Heart Assoc 8(12): e012219. 10.1161/JAHA.119.012219
- Li D, Yang S, Xing Y, Pan L, Zhao R, Zhao Y, Liu L, Wu M (2021). Novel Insights and Current Evidence for Mechanisms of Atherosclerosis: Mitochondrial Dynamics as a Potential Therapeutic Target. Front Cell Dev Biol 9: 673839. 10.3389/fcell.2021.673839
- Chen X, Zhang D, Li Y, Wang W, Bei W, Guo J (2021). NLRP3 inflammasome and IL-1beta pathway in type 2 diabetes and atherosclerosis: Friend or foe? Pharmacol Res 173: 105885. 10.1016/j.phrs.2021.105885
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ (2013). CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14(8): 812-820. 10.1038/ni.2639
- Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK (2010). Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5(7): e11765. 10.1371/journal.pone.0011765
- Tall AR, Westerterp M (2019). Inflammasomes, neutrophil extracellular traps, and cholesterol. J Lipid Res 60(4): 721-727. 10.1194/jlr.S091280
- Thacker SG, Zarzour A, Chen Y, Alcicek MS, Freeman LA, Sviridov DO, Demosky SJ, Remaley AT (2016). High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 149(3): 306-319. 10.1111/imm.12638
- Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G (2013). K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38(6): 1142-53. 10.1016/j.immuni.2013.05.016
- Karasawa T, Kawashima A, Usui-Kawanishi F, Watanabe S, Kimura H, Kamata R, Shirasuna K, Koyama Y, Sato-Tomita A, Matsuzaka T, Tomoda H, Park SY, Shibayama N, Shimano H, Kasahara T, Takahashi M (2018). Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arterioscler Thromb Vasc Biol 38(4): 744-756. 10.1161/ATVBAHA.117.310581
- Anzai F, Karasawa T, Komada T, Yamada N, Miura Y, Sampilvanjil A, Baatarjav C, Fujimura K, Matsumura T, Tago K, Kurosu H, Takeishi Y, Kuro-O M, Takahashi M (2021). Calciprotein Particles Induce IL-1beta/alpha-Mediated Inflammation through NLRP3 Inflammasome-Dependent and -Independent Mechanisms. Immunohorizons 5(7): 602-614. 10.4049/immunohorizons.2100066
- Yang Y, Wang H, Kouadir M, Song H, Shi F (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 10(2): 128. 10.1038/s41419-019-1413-8
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP (2011). Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12(5): 408-415. 10.1038/ni.2022
- Stachon P, Heidenreich A, Merz J, Hilgendorf I, Wolf D, Willecke F, von Garlen S, Albrecht P, Hardtner C, Ehrat N, Hoppe N, Reinohl J, von Zur MC, Bode C, Idzko M, Zirlik A (2017). P2X7 Deficiency Blocks Lesional Inflammasome Activity and Ameliorates Atherosclerosis in Mice. Circulation 135(25): 2524-2533. 10.1161/CIRCULATIONAHA.117.027400
- Peng K, Liu L, Wei D, Lv Y, Wang G, Xiong W, Wang X, Altaf A, Wang L, He D, Wang H, Qu P (2015). P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med 35(5): 1179-1188. 10.3892/ijmm.2015.2129
- Shokoples BG, Paradis P, Schiffrin EL (2021). P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease. Arterioscler Thromb Vasc Biol 41(1): 186-199. 10.1161/ATVBAHA.120.315116
- Folco EJ, Sukhova GK, Quillard T, Libby P (2014). Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res 115(10): 875-883. 10.1161/CIRCRESAHA.115.304437
- Watanabe S, Usui-Kawanishi F, Karasawa T, Kimura H, Kamata R, Komada T, Inoue Y, Mise N, Kasahara T, Takahashi M (2020). Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages. J Cell Physiol 235(10): 7554-7566. 10.1002/jcp.29659
- Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ (2017). Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A 114(18): 4763-4768. 10.1073/pnas.1620458114
- Ding Z, Liu S, Wang X, Dai Y, Khaidakov M, Deng X, Fan Y, Xiang D, Mehta JL (2014). LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: implications in atherogenesis. Cardiovasc Res 103(4): 619-628. 10.1093/cvr/cvu114
- Tumurkhuu G, Shimada K, Dagvadorj J, Crother TR, Zhang W, Luthringer D, Gottlieb RA, Chen S, Arditi M (2016). Ogg1-Dependent DNA Repair Regulates NLRP3 Inflammasome and Prevents Atherosclerosis. Circ Res 119(6): e76-90. 10.1161/CIRCRESAHA.116.308362
- Suarez-Rivero JM, Pastor-Maldonado CJ, Povea-Cabello S, Alvarez-Cordoba M, Villalon-Garcia I, Talaveron-Rey M, Suarez-Carrillo A, Munuera-Cabeza M, Sanchez-Alcazar JA (2021). From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 9(3): 258. 10.3390/biomedicines9030258
- Yuan J, Najafov A, Py BF (2016). Roles of Caspases in Necrotic Cell Death. Cell 167(7): 1693-1704. 10.1016/j.cell.2016.11.047
- Li J, Zhang Y, Zhang L, An Z, Song J, Wang C, Ma Y, Gu Q, Luo Q, Yang W, Du Y, Wu W (2021). Fine particulate matter exposure exacerbated nasal mucosal damage in allergic rhinitis mice via NLRP3 mediated pyroptosis. Ecotoxicol Environ Saf 228: 112998. 10.1016/j.ecoenv.2021.112998
- Li L, Xing C, Zhou J, Niu L, Luo B, Song M, Niu J, Ruan Y, Sun X, Lei Y (2021). Airborne particulate matter (PM2.5) triggers ocular hypertension and glaucoma through pyroptosis. Part Fibre Toxicol 18(1): 10. 10.1186/s12989-021-00403-4
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575): 660-665. 10.1038/nature15514
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610): 153-158. 10.1038/nature18629
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535(7610): 111-116. 10.1038/nature18590
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526(7575): 666-671. 10.1038/nature15541
- Kayagaki N, Warming S, Lamkanfi M, Vande WL, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM (2011). Non-canonical inflammasome activation targets caspase-11. Nature 479(7371): 117-121. 10.1038/nature10558
- Ji N, Qi Z, Wang Y, Yang X, Yan Z, Li M, Ge Q, Zhang J (2021). Pyroptosis: A New Regulating Mechanism in Cardiovascular Disease. J Inflamm Res 14(2647-2666. 10.2147/JIR.S308177
- Zeng C, Wang R, Tan H (2019). Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int J Biol Sci 15(7): 1345-1357. 10.7150/ijbs.33568
- Geovanini GR, Libby P (2018). Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond) 132(12): 1243-1252. 10.1042/CS20180306
- Wolf D, Ley K (2019). Immunity and Inflammation in Atherosclerosis. Circ Res 124(2): 315-327. 10.1161/CIRCRESAHA.118.313591
- Jia C, Chen H, Zhang J, Zhou K, Zhuge Y, Niu C, Qiu J, Rong X, Shi Z, Xiao J, Shi Y, Chu M (2019). Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol 67(311-318. 10.1016/j.intimp.2018.12.028
- Wang Y, Liu X, Shi H, Yu Y, Yu Y, Li M, Chen R (2020). NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med 10(1): 91-106. 10.1002/ctm2.13
- Libby P, Ridker PM, Hansson GK (2011). Progress and challenges in translating the biology of atherosclerosis. Nature 473(7347): 317-325. 10.1038/nature10146
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgozoglu L, Lewis EF (2019). Atherosclerosis. Nat Rev Dis Primers 5(1): 56. 10.1038/s41572-019-0106-z
- Botts SR, Fish JE, Howe KL (2021). Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front Pharmacol 12: 787541. 10.3389/fphar.2021.787541
- Gimbrone MJ, Garcia-Cardena G (2016). Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 118(4): 620-636. 10.1161/CIRCRESAHA.115.306301
- Mestas J, Ley K (2008). Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 18(6): 228-232. 10.1016/j.tcm.2008.11.004
- Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, Tilley DG, Monroy MA, Choi ET, Thomas CJ, Jiang X, Wang H, Yang XF (2015). Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol 35(4): 804-816. 10.1161/ATVBAHA.115.305282
- Moore KJ, Sheedy FJ, Fisher EA (2013). Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13(10): 709-721. 10.1038/nri3520
- Martinet W, Coornaert I, Puylaert P, De Meyer G (2019). Macrophage Death as a Pharmacological Target in Atherosclerosis. Front Pharmacol 10: 306. 10.3389/fphar.2019.00306
- Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, Xiao T, Liu W, Thomas DG, Hajebrahimi MA, Pircher J, Silvestre-Roig C, Kotini AG, Luchsinger LL, Wei Y, Westerterp M, Snoeck HW, Papapetrou EP, Schulz C, Massberg S, Soehnlein O, Ebert B, Levine RL, Reilly MP, Libby P, Wang N, Tall AR (2021). The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592(7853): 296-301. 10.1038/s41586-021-03341-5
- Bentzon JF, Otsuka F, Virmani R, Falk E (2014). Mechanisms of plaque formation and rupture. Circ Res 114(12): 1852-1866. 10.1161/CIRCRESAHA.114.302721
- Bennett MR, Sinha S, Owens GK (2016). Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 118(4): 692-702. 10.1161/CIRCRESAHA.115.306361
- Mayr M, Xu Q (2001). Smooth muscle cell apoptosis in arteriosclerosis. Exp Gerontol 36(7): 969-987. 10.1016/s0531-5565(01)00090-0
- Mensah GA (2007). Healthy endothelium: the scientific basis for cardiovascular health promotion and chronic disease prevention. Vascul Pharmacol 46(5): 310-314. 10.1016/j.vph.2006.10.013
- Yan ZQ, Hansson GK (2007). Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev 219: 187-203. 10.1111/j.1600-065X.2007.00554.x
- He X, Fan X, Bai B, Lu N, Zhang S, Zhang L (2021). Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol Res 165: 105447. 10.1016/j.phrs.2021.105447
- Li Y, Niu X, Xu H, Li Q, Meng L, He M, Zhang J, Zhang Z, Zhang Z (2020). VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp Cell Res 389(1): 111847. 10.1016/j.yexcr.2020.111847
- Pan J, Han L, Guo J, Wang X, Liu D, Tian J, Zhang M, An F (2018). AIM2 accelerates the atherosclerotic plaque progressions in ApoE-/-mice. Biochem Biophys Res Commun 498(3): 487-494. 10.1016/j.bbrc.2018.03.005
- Cao Z, Huang D, Tang C, Lu Y, Huang S, Peng C, Hu X (2022). Pyroptosis in diabetes and diabetic nephropathy. Clin Chim Acta 531: 188-196. 10.1016/j.cca.2022.04.011
- Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M, Meng Q, Zhou B, Leng Y, Xia ZY (2017). NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxid Med Cell Longev 2017: 9743280. 10.1155/2017/9743280
- Wang S, Yuan YH, Chen NH, Wang HB (2019). The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol 67: 458-464. 10.1016/j.intimp.2018.12.019
- Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T (2020). Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging (Albany NY) 12(10): 9405-9426. 10.18632/aging.103215
- Li S, Sun Y, Song M, Song Y, Fang Y, Zhang Q, Li X, Song N, Ding J, Lu M, Hu G (2021). NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 6(23): e146852. 10.1172/jci.insight.146852
- Ran Y, Su W, Gao F, Ding Z, Yang S, Ye L, Chen X, Tian G, Xi J, Liu Z (2021). Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-kappaB Suppression and NLRP3 Inflammasome Inhibition. Oxid Med Cell Longev 2021: 1552127. 10.1155/2021/1552127
- Zeng W, Wu D, Sun Y, Suo Y, Yu Q, Zeng M, Gao Q, Yu B, Jiang X, Wang Y (2021). The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci Rep 11(1): 19305. 10.1038/s41598-021-98437-3
- Coll RC, Schroder K, Pelegrin P (2022). NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci 43(8): 653-668. 10.1016/j.tips.2022.04.003
- Song D, Li M, Yu X, Wang Y, Fan J, Yang W, Yang L, Li H (2022). The Molecular Pathways of Pyroptosis in Atherosclerosis. Front Cell Dev Biol 10: 824165. 10.3389/fcell.2022.824165
- Swanson KV, Deng M, Ting JP (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8): 477-489. 10.1038/s41577-019-0165-0
–
AUTHOR CONTRIBUTIONS
Xiang Zeng: Writing – Original draft, Conceptualization, Supervision, Funding acquisition. Dongling Liu: Writing – review & editing, Conceptualization, Validation, Funding acquisition. Xia Huo: Writing – review & editing, Valida-tion. Yue Wu: Writing – review & editing, Validation. Cui-qing Liu: Writing – review & editing, Validation, Funding acquisition. Qinghua Sun: Writing – review & editing, Validation, Supervision. All authors have read and ap-proved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the grants from the Open Project Program of the First Affiliated Hospital of Xinxiang Medical University (XZZX2022012), the National Natural Science Foundation of China (81973001), and the Postdoc-toral Science Foundation of China (2020M683183).
COPYRIGHT
© 2022

Pyroptosis in NLRP3 inflammasome-related atherosclerosis by Zeng et al. is licensed under a Creative Commons Attribution 4.0 International License.