Back to article: Mitochondrial dynamics links PINCH-1 signaling to proline metabolic reprogramming and tumor growth
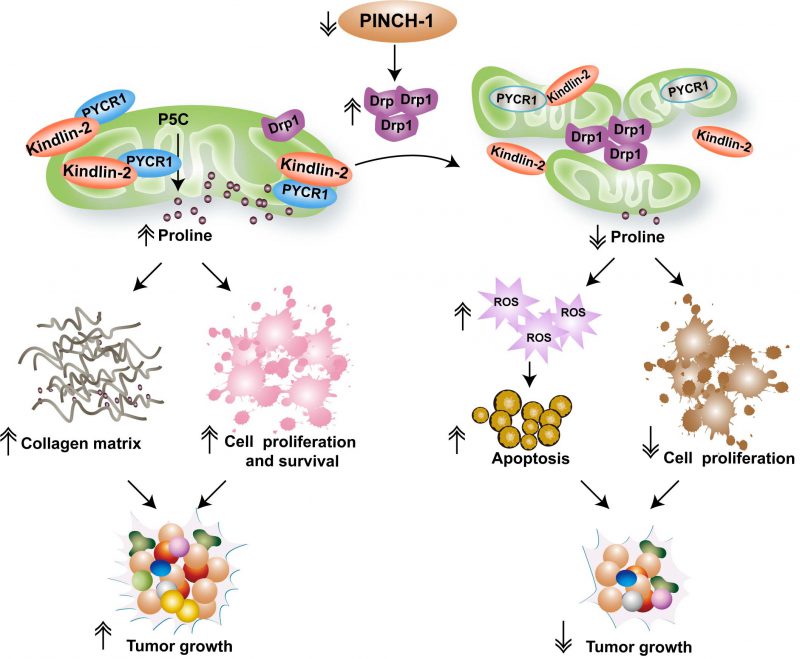
FIGURE 1: A model of PINCH-1-mediated regulation of mitochondrial dynamics and proline synthesis. The figure depicts a model in which mitochondrial dynamics links PINCH-1 signaling to proline metabolic reprogramming and tumor growth. Cells in cancerous tissues express an elevated level of PINCH-1, which suppresses Drp1 expression and prevents excessive mitochondrial fragmentation. PINCH-1 deficiency increases Drp1 expression, resulting in excessive mitochondrial fragmentation, which in turn inhibits kindlin-2 mitochondrial translocation and interaction with PYCR1. Inhibition of kindlin-2 interaction with PYCR1 promotes proteolytic degradation of PYCR1 and consequently reduces PYCR1 level and proline synthesis. Inadequacy of proline synthesis in cancer cells reduces cell proliferation, increases reactive oxygen species (ROS) signaling and promotes apoptosis. Furthermore, reduced proline synthesis in collagen matrix-producing cells (e.g., cancer-associated fibroblasts) restrict tumor-associated fibrosis. Collectively, these effects contribute to suppression of tumor fibrosis and growth in response to inhibition of PINCH-1 signaling.