Back to article: AMPK maintains TCA cycle through sequential phosphorylation of PDHA to promote tumor metastasis
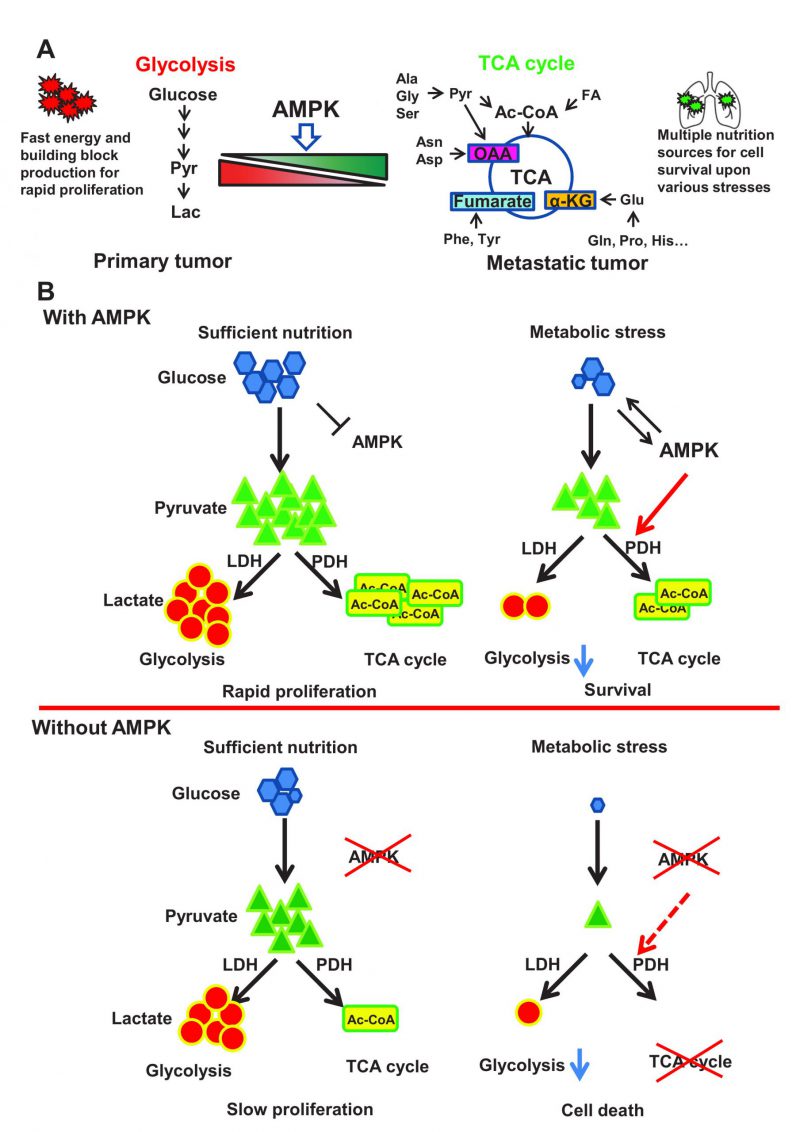
FIGURE 1: AMPK protects cancer cells from stresses induced cell death through rewiring cellular metabolism towards TCA cycle, thus facilitating metastasis. (A) Primary tumor largely relies on glycolysis to fulfill the energy and building block needs for rapid proliferation. While metastatic tumor frequently encounters diverse stresses including metabolic stress especially during colonization process in the secondary site with hostile microenvironments. Metabolic shift from glycolysis towards TCA cycle enables disseminated cancer cells to utilize broad range of nutrition sources for survival. AMPK critically regulates the activity of pyruvate dehydrogenase complex, thus favoring pyruvate metabolism towards TCA cycle and facilitating tumor metastasis. Pyr: Pyruvate; Lac: Lactate; Ala: Alanine; Gly: Glycine; Ser: Serine; Ac-CoA: Acetyl-CoA; FA: Fatty Acid; Asn: Asparagine; Asp: Aspartate; OAA: Oxaloacetate; Phe: Phenylalanine; Tyr: Tyrosine; α-KG: α-Ketoglutarate; Glu: Glutamate; Gln: Glutamine; Pro: Proline; His: Histidine. (B) A schematic model illustrates the role of AMPK-PDH axis in protecting cancer cells from metabolic stress-induced cell death. Under sufficient nutrition conditions, cancer cells can use either glycolysis or oxidative phosphorylation to maintain the energy and building blocks for rapid proliferation. AMPK is activated upon metabolic stress to trigger activation of the PDH complex, which then converts pyruvate to Ac-CoA to facilitate TCA cycle. This AMPK mediated metabolic reprogramming process will allow cancer cells to utilize energy and building block generated from TCA cycle for cell survival under metabolic stress. AMPK deficient cancer cells are highly reliable on glycolysis due to the defects in PDH activity and TCA cycle. Under sufficient nutrition conditions, these cells could utilize glycolysis to maintain cell survival and proliferation, but fail to use oxidative phosphorylation for survival under metabolic stress due to the defect in TCA cycle.