Back to article: Regulation of T cell antitumor immune response by tumor induced metabolic stress
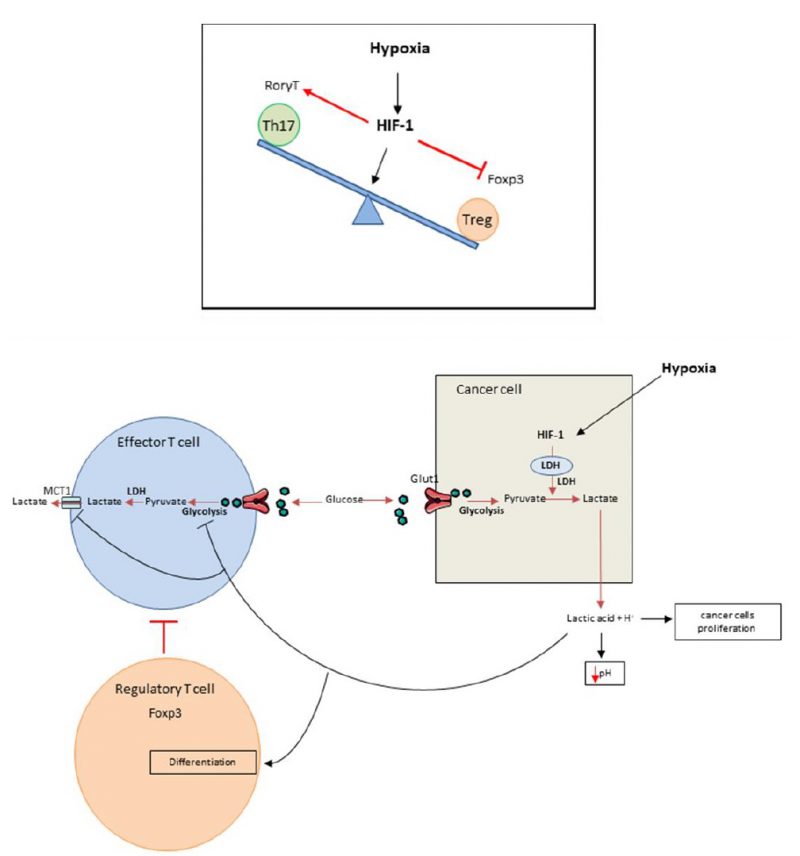
FIGURE 1. Top: Role of hypoxia in Th17/Treg balance disregulation. Hypoxia within the tumors enhances the Th17 development over the Treg development. This is due to the induction of HIF-1 that will in one side promote the expression of ROR?t through its cooperation with STAT3 and in the other side bind to Foxp3 inducing its ubiquitination and degradation. Bottom: Hypoxia promotes immunosuppression. Cancer cells have a high glycolytic activity manifested by glucose intake through Glut1 transporter that is metabolized into pyruvate and then into lactic acid through LDH activation induced by HIF-1. The lactic acid is exported within the tumor microenvironment and induces its acidification. Effector T lymphocytes are also dependent of their glycolytic activity and must release lactate by SLC16A1; best known as MCT1. In this context of acidification, MCT1 is inhibited thus blocking the glycolysis and consequently the activation of the effector T cells. Moreover the high lactate concentration within the tumor microenvironment will promote Treg biology by inducing FoxP3 expression. In these conditions hypoxia leads to immunosuppression through the activation or inhibition of different immune cells populations.