Back to article: Systemic signalling and local effectors in developmental stability, body symmetry, and size
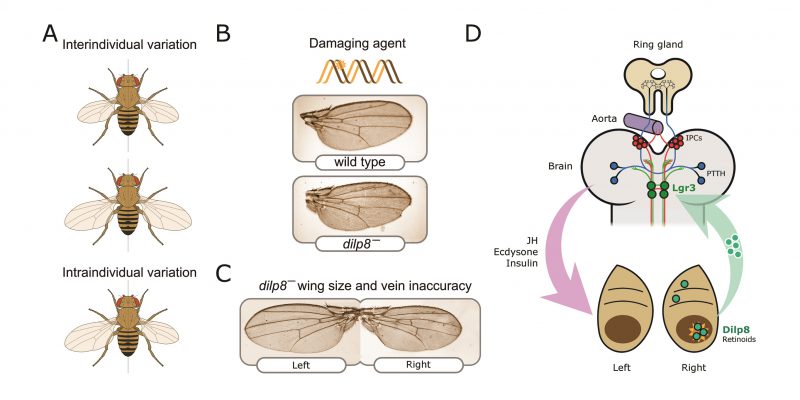
FIGURE 2: Neuroendocrine control of symmetry and body size. (A) Loss of the Dilp8 hormone yields flies with variable body sizes (inter-individual) and increased bilateral asymmetry (intraindividual variability). (B) Feeding Drosophila juveniles with the DNA-damaging agent ethyl methanosulfonate (EMS) induces massive cell death in the imaginal discs, cell cycle arrest, and strong developmental delay. Without dilp8 (bottom wing), EMS-fed animals cannot recover from this damage and exhibit a 6-fold increase in pattern and growth inaccuracies [14]. (C) dilp8 mutants exhibit left-right wing asymmetry and also pattern inaccuracies, which may reflect the unmasking of pre-existing or acquired mutations, stochastic noise, and/or the negative effect of the environment (e.g., temperature stress). (D) Dilp8 produced by damaged or growth-perturbed cells, which also activates the production of other ‘alarm' signals such as retinoid signals [145] is released to circulation and acts in the brain through the relaxin receptor Lgr3 (green neurons). Lgr3 co-regulates two neuronal populations which control growth and maturation rate by acting on the ring gland. The ring gland is a central neuroendocrine organ regulating organismal growth rate and timing of maturation [45]. Distinct groups of cells within the ring gland — the corpus allatum — produce the juvenile hormone (JH), and cells of the prothoracic gland synthesise and release the steroid hormone ecdysone. Its complex functions are centrally controlled by neurons that produce the prothoracicotropic hormone (PTTH; represented as blue circles) [139, 146] and the insulin-producing cells (IPCs; red circles). IPCs produce insulin-like peptides, primarily Dilp2, Dilp3, and Dilp5, and regulate systemic growth and ecdysone biosynthesis (reviewed in [47].
14. Garelli A, Gontijo AM, Miguela V, Caparros E, and Dominguez M (2012). Imaginal Discs Secrete Insulin-Like Peptide 8 to Mediate Plasticity of Growth and Maturation. Science 336(6081): 579–582. doi: 10.1126/science.1216735
45. Gokhale RH, and Shingleton AW (2015). Size control: the developmental physiology of body and organ size regulation: The developmental physiology of size control. Wiley Interdiscip Rev Dev Biol 4(4): 335–356. doi: 10.1002/wdev.181
47. Andersen DS, Colombani J, and Léopold P (2013). Coordination of organ growth: principles and outstanding questions from the world of insects. Trends Cell Biol 23(7): 336–344. doi: 10.1016/j.tcb.2013.03.005
139. Stern DL, Emlen DJ (1999). The developmental basis for allometry in insects. Development 126(6): 1091–1101. PMID: 10021329
145. Halme A, Cheng M, and Hariharan IK (2010). Retinoids Regulate a Developmental Checkpoint for Tissue Regeneration in Drosophila. Curr Biol 20(5): 458–463. doi: 10.1016/j.cub.2010.01.038
146. Nijhout HF, and Callier V (2015). Developmental Mechanisms of Body Size and Wing-Body Scaling in Insects. Annu Rev Entomol 60(1): 141–156. doi: 10.1146/annurev-ento-010814-020841