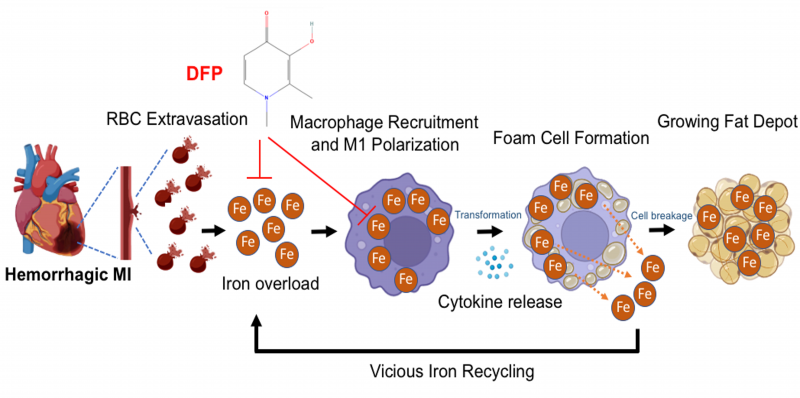
FIGURE 2: A proposed scheme of how hemorrhagic myocardial infarction (MI) induces iron accumulation, proinflammatory response and lipomatous metaplasia. In hemorrhagic myocardial infarction (MI), the extravasated red blood cells (RBCs) lead to iron overload and promote the recruitment of unpolarized macrophages to the infarction zone. This iron when taken up by macrophages induce their polarization into pro-inflammatory state and subsequently transformation into foam cells through disproportionate lipid influx and cholesterol accumulation. As the disease progresses, iron is released through foam cell degradation (apoptosis), which enters a vicious cycle that perpetuates to continually support inflammation and leads to the growth of the fat depot in the infarction zone. These detrimental events contribute to functional and structural losses of the heart, which define chronic heart failure. Timely application of an intra- and extra-cellular ferric iron chelator deferiprone (DFP) can markedly reduce iron and fat with the MI zone. Figure was generated using Biorender.