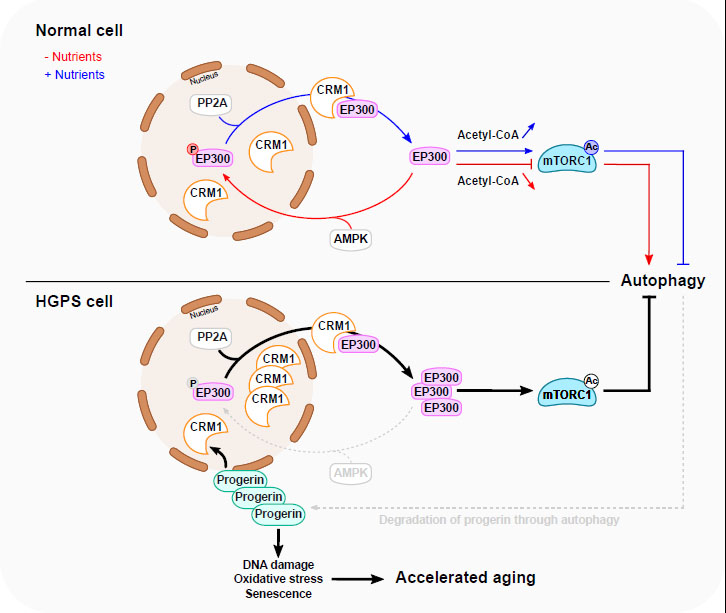
FIGURE 1: A proposed model for EP300 cytoplasm-nucleus shuttling in response to nutrients and in HGPS. Nutrient depletion triggers the relocation of EP300 from the cytoplasm to the nucleus resulting in the inhibition of mTORC1 and subsequent stimulation of autophagy. This is facilitated through AMPK-dependent phosphorylation of EP300 at Serine 89. Nutrients replenishment in starved cells induces PP2A-dependent dephosphorylation of nuclear EP300, facilitating its transportation to cytoplasm by CRM1, thereby promoting mTORC1 reactivation. In HGPS cells, EP300 cytoplasm–nucleus shuttling is altered, causing mTORC1 hyperactivation and impaired autophagy. This is mediated by Progerin-dependent upregulation of CRM1 and alterations in AMPK activity. Interestingly, impaired autophagy leads, in turn, to the accumulation of Progerin, which potentially amplifies the disturbance of the mTORC1/autophagy pathway. The accumulation of Progerin initiates diverse cellular responses linked to accelerated aging. Normalizing HGPS phenotypes can be achieved by modulating EP300 nucleocytoplasmic shuttling. Blue line depicts a nutrient-rich condition, while the red line depicts a nutrient-depleted condition. The grey dotted lines delineate the compromised autophagy process observed in HGPS.