Research Articles:
Cell Stress, Vol. 8, No. 1, pp. 112 - 124; doi: 10.15698/cst2024.11.301
Endogenous plasma resuspension of peripheral blood mononuclear cells prevents preparative-associated stress that modifies polyA-enriched RNA responses to subsequent acute stressors
1 National Heart and Lung Institute, Imperial College London, W12 ONN, London, UK. 2 National Institute for Health Research (NIHR) Imperial Biomedical Research Centre, W2 1NY, London, UK. 3 Pharmacy, Imperial College Healthcare NHS Trust, W12 OHS, London, UK. 4 NIHR Imperial BRC Genomic Facility, Faculty of Medicine, Imperial College London. 5 Specialist Medicine, Imperial College Healthcare NHS Trust, W12 OHS, London, UK.
Keywords: Cycloheximide, hypothermia, integrated stress response, intronless (single exon) genes, iron, protein translation inhibition, reactive oxygen species, poly-A selected RNASeq, experimental variability.
Abbreviations:
ATF - activating transcription factor,
eiF2 - eukaryotic initiation factor 2,
GO - gene ontology,
NMD - nonsense-mediated decay,
PBMC - peripheral blood mononuclear cell,
TNF - tumor necrosis factor.
Received originally: 11/10/2023 Received in revised form: 12/09/2024
Accepted: 03/10/2024
Published: 28/11/2024
Correspondence:
Claire L Shovlin, PhD FRCP, Professor of Practice (Clinical and Molecular Medicine), National Heart and Lung Institute, Imperial College London, Hammersmith Campus, Du Cane Road, London W12 0NN, UK.; c.shovlin@imperial.ac.uk
Conflict of interest statement: The authors have no direct conflict of interest to declare. The use of MEK inhibitors for treatment of bleeding in hereditary haemorrhagic telangiectasia (HHT) is the subject of a patent application by Imperial College London.
Please cite this article as: Dongyang Li, Karina Al-Dahleh, Daniel A Murphy, Sonya Georgieva, Nik Matthews, Claire L Shovlin (2024). Endogenous plasma resuspension of peripheral blood mononuclear cells prevents preparative-associated stress that modifies polyA-enriched RNA responses to subsequent acute stressors. Cell Stress 11: 112-124. doi: 10.15698/cst2024.11.301
Abstract
Human peripheral blood mononuclear cells (PBMCs) are used to examine biological processes and disease, when basal variability in cellular activation and splicing is described and unexplained. Using isolation systems that maintained buffy coat cells (PBMCs, platelets) in their own plasma, poly-A enriched RNA-sequencing (RNASeq) detected 42,720 Ensembl gene IDs, including >95% of the top 100 Genotype Tissue Expression Project (GTEx)-expressed genes in lung, colon, heart, skeletal muscle and liver, and 10/17 clinically-actionable genes listed by the Pharmacogenomics Knowledgebase. Transcriptome changes were defined after 1h treatment with 32◦C hypothermia (hsp70 family member change), 10 µmol/L ferric citrate that had no discernible effect, and 100 µg/mL cycloheximide leading to induction of primary response (immediate early) genes including IL1B and TNF. Same-donor PBMCs prepared conventionally using washes then resuspension in serum-supplemented media demonstrated basal upregulation of stress signalling pathway genes that masked and overlapped differential gene expression profiles after 100 µg/L cycloheximide. Plasma-resuspended PBMCs demonstrated minor transcriptome changes after 40 µmol/L ferric citrate, whereas consistent and greater magnitude changes were observed for washed/media- resuspended PBMCs. We conclude that endogenous plasma-maintained PBMCs provide a more robust platform to interrogate acute cellular perturbations trig- gering innate immunity, and that varying susceptibility of PBMCs to preparative stresses is an important component of experimental variability.
INTRODUCTION
Cells are impacted by complex and co-occurring stresses throughout a life-course. Our goal was to generate physiologically relevant models of acute cellular stress, initially in relation to 3’ untranslated regions (3’UTRs), as these can be used differentially under stress conditions, 1 and contained impact-predicted DNA variants 2. In selecting a suitable cell type for the rapid characterisation of patient-derived cells, we focussed on peripheral blood mononuclear cells (PBMCs) which are readily accessible, as derived from a peripheral venous blood sample. While PBMCs mediate diverse inflammatory and thrombotic processes 3, 4, 5, our validations of DNA variants relevant to endothelial pathologies 2 led us to hypothesise they could evaluate broader functional and pathophysiological questions.
Recent studies demonstrate that between-sample variability is generated during PBMC isolation 6, 7, 8, including upregulation of genes regulated by NF-κB in response to tumour necrosis factor (TNF), 7 and differential splicing effects detectable at 2hr 8. Recognition of this unexplained variability has led to calls to place PBMC processing facilities close to patient recruitment sites 9. Previous experience maintaining endothelial cell health 10, 11, 12, 13 led us to consider whether PBMC preparation methods themselves might be resulting in uncontrolled, mild cellular stress. Standard PBMC isolation techniques dilute whole blood in an equal volume of phosphate-buffered saline (PBS) prior to first centrifugation, and incorporate multiple PBS wash steps before resuspension in serum-supplemented media. To maintain PBMCs in an environment closer to their native state when multiple buffers and growth factors protect cells, we developed an isolation and test system where the buffy coat is resuspended and remains in its own plasma prior to, during, and after experimental treatments, and for final centrifugation 2.
Our goal was not only to compare plasma and media-resuspended PBMC transcriptomes at baseline, but also changes after experimental strong and weak 1h stresses. As a strong stress, we selected a model of the common consequence from the integrated stress response, when cells respond not only by activation of specific survival programmes, but also by transient inhibition of global protein synthesis 14, 15, 16. In unstressed cells, translation initiation proceeds because the alpha subunit of eukaryotic initiation factor 2 (eIF2α) is maintained in an unphosphorylated form by protein phosphatase 1C (PPP1C): this enables the eIF2 beta subunit (eIF2β) to exchange GDP for GTP, facilitating assembly of the charged methionyl-initiator tRNA eIF∙GTP∙methionyl-initiator tRNA ternary complex 17, 18. In contrast, in stressed cells, E1F2α is phosphorylated at serine 51 which prevents eIF2β GDP-GTP exchange, and therefore attenuates global translation initiation. Experimentally, cycloheximide can be used to inhibit protein translation by binding to the 60S ribosomal subunit E-site 19, 20, and cycloheximide-chase assays are used routinely to measure protein stability 18, 21. Secondary consequences of cycloheximide are also exploited experimentally, for example, inhibition of nonsense-mediated decay (NMD) 22, 23 which is translation-dependent 24. Diverse cellular stresses are known to inhibit NMD 25, 26 while the integrated stress response itself depends upon key natural NMD targets such as activating transcription factor (ATF)3 and ATF4 14, 15, 25, 26, 27.
Two weaker cellular stresses were selected for evaluation. We tested 10 and 40 µmol/L ferric citrate which are in the range of concentrations shown to induce changes in endothelial cells in vitro 11, 28, and an incubation temperature of 32°C, noting cell culture manipulations are usually performed at ambient temperatures of ≤25°C. Both stresses are relevant to human health. Total serum iron exhibits diurnal variability and responds rapidly to gastrointestinal iron load. In mice, the peak-trough difference in mean serum iron is 12 μmol/L 29, while plasma levels of non-transferrin bound iron reach or exceed 10 µmol/L after conventional iron tablets to treat anaemia in humans 30, 31, 32, 33, 34. Our interest in therapeutic iron had been stimulated because approximately one in 20 patients with a rare vascular disorder reported that their iron tablets used to treat anaemia provoked acute nosebleeds35, 36, 37. Cold is a biological stressor, and 32°C delimits mild from moderate hypothermia38. Cold is also used in clinical practice to reduce cell injury39, 40.
Here we present global RNASeq results that support buffy coat/PBMCs as an investigative tool across broad RNA species; reveal that 1hr inhibition of protein translation by cycloheximide leads to a primary response (immediate early) gene signature that is partially masked and mirrored by PBMC activation during standard preparative methods, and that plasma-resuspended PBMCs were more resilient to iron stress than PBMCs that had been prepared by more conventional methods.
RESULTS
Broad relevance of PBMCs as a differential RNA expression system
First, we considered the extent to which the buffy coat/PBMC system could be a model for gene expression beyond the usual inflammatory-thrombotic foci. Across three donors, 27,569 of the 57,500 Ensembl gene IDs tested demonstrated a mean basal expression of at least one (see upper graph, Figure 1A). For non-blood tissues, we examined expression of the top 100 transcripts for lung, heart, colon, skeletal muscle and liver in the Genotype Tissue Expression (GTEx) Project 41. As shown in Figure 1B, 95-100% were expressed in the buffy coat/PBMC system according to the tissue. To explore relevance to common gene variants important in clinical practice, we examined the expression of all genes with actionable pharmacogenomic guidance according to the Pharmacogenomics Knowledgebase 42, Dutch Pharmacogenetics Working Group 43, and Clinical Pharmacogenetics Implementation Consortium 44. Ten of the 17 actionable Pharmacogenes demonstrated modest to high expression in the buffy coat/PBMCs (Figure 1C).
–
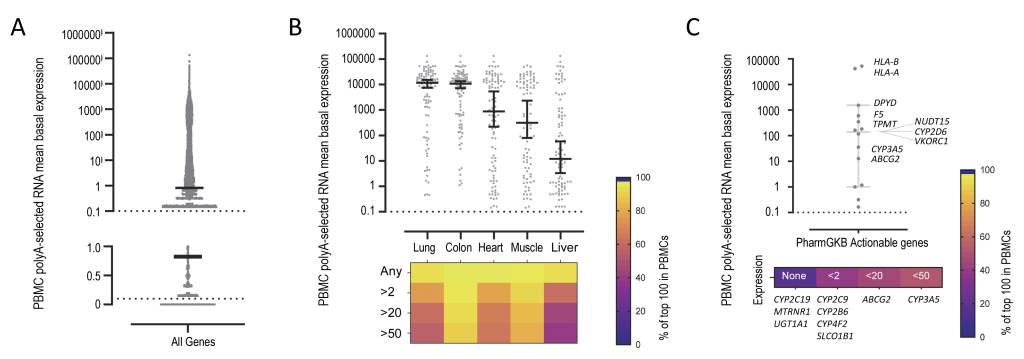 |
FIGURE 1: Extended relevance of human peripheral blood mononuclear cell (PBMC) test system . Polyadenylated-transcript enriched expression in human PBMCs under basal conditions, from three volunteer donors and 2 replicates/donor [median, 95% confidence intervals]. (A) All Ensembl [45] gene IDs (N=57,500 coding and non-coding gene IDs). The upper graph plots the 42,720 (74.3%) with mean basal expression >0.1 in PBMCs (logarithmic scale), and lower graph, the 29,931 (52.1%) with mean basal expression <1.0 (linear scale). Median and 95% confidence interval indicated. (B) PBMC expression of the 100 most expressed genes in five of the Genotype Tissue Expression (GTEx) project [41] organ-based tissues: 95% of lung, 100% of sigmoid colon; 99% of heart (atrial appendage), 98% of skeletal muscle and 95% of liver genes in the top 100 expression in the respective GTEx tissues were expressed in the PBMCs. The upper graph plots individual expression values, median and 95% confidence intervals; the lower graph is a heat map indicating the percentage (%) of the transcripts for each tissue expressed at the specified level. (C) The 17 actionable Pharmacogenes according to the Pharmacogenomics Knowledgebase [42], based on recommendations from the Dutch Pharmacogenetics Working Group [43], and Clinical Pharmacogenetics Implementation Consortium [44]. The upper graph plots individual expression values with median and 95% confidence intervals, annotated by gene identity; the lower graph is a heat map indicating the percentage (%) of the transcripts expressed at the specified level, with the individual genes in each expression category listed. Note the high PBMC expression of ABCG2, CYP2D6, CYP3A5, DPYD, F5, HLA-A, HLA-B, NUDT15, TPMT and VKORC1. |
PBMC isolation using plasma resuspension does not activate PBMCs
Next, we examined RNASeq expression profiles in polyadenylated RNA-enriched libraries derived from the buffy coat cells that had remained in their endogenous plasma throughout isolation, experimental prewarming to 37°C, 1hr experimental treatments and final centrifugation before lysis in TRI reagent (Cambridge Bioscience, UK), and immediate freezing at -80°C. In supervised analyses, we examined raw RNASeq alignments to major driver genes for cellular stress where basal expression in unstressed PBMCs should be low. For TNF 7, IL1B 46, FOS 47, and ATF3 48, expression was very low following 1hr control incubation at 37°C, and also, after 1hr treatment with 10 μmol/L ferric citrate, or 1hr at 32°C (Figure 2A). However, there was a marked increase in alignments to all four genes following 1hr cycloheximide 100 μg/mL (Figure 2A). We concluded that the isolation system was not activating PBMCs, and turned to unsupervised analyses.
–
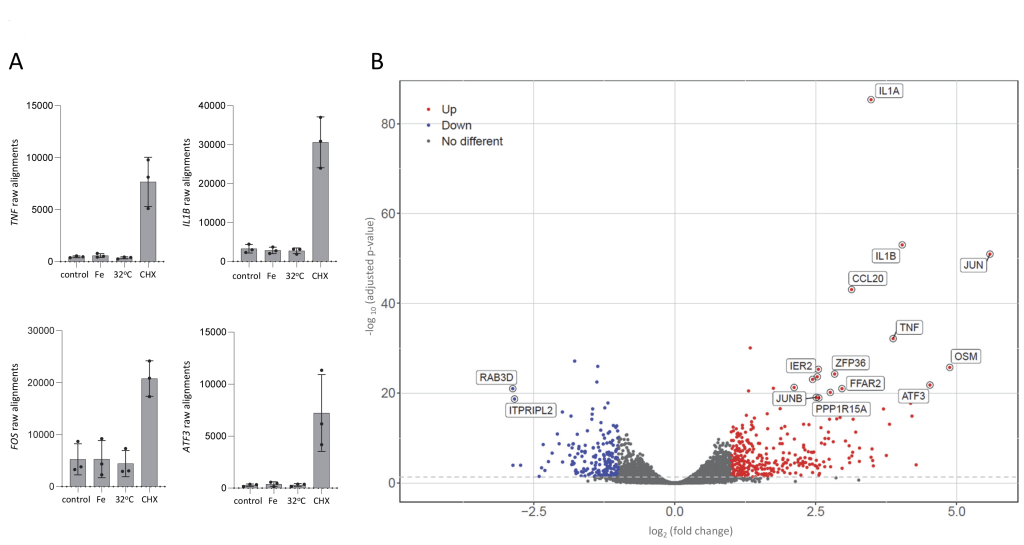 |
FIGURE 2: Differential gene expression in human peripheral blood mononuclear cells (PBMCs) after 1h stress treatments. (A) Raw alignments in polyA-selected RNASeq libraries generated from PBMCs from donors 1-3 after 1hr in control and experimental treatments. 32◦C was the temperature used to model mild/moderate hypothermia; Fe, 10 µmol/L ferric citrate to provide a mild oxidative stress; CHX, 100 µg/mL cycloheximide to inhibit protein translation and model the common output of the integrated stress response. TNF encodes TNFα [7]; IL1B encodes interleukin-1β [46]; FOS encodes c-FOS [47]; ATF3 encodes activating transcription factor (ATF)3 [49]. Mean, standard deviation and individual values (N=3, from donors 1-3) are displayed. (B) Volcano plot of genes differentially expressed after 1h cycloheximide. Thresholds for the volcano plot of genes differentially expressed to adjusted p<0.05 were log2(fold change) >1 (red) and <1 (blue). |
Transcriptome changes after 1h stresses
Quality scores did not differ between libraries from control and treated samples (Supplementary Figure S1).
After 1hr incubation at 32°C, a single gene was differentially expressed to Benjamini p<0.05 (Supplementary Table S1). HSPA1B, an intronless gene encoding heat shock protein family A (hsp70) member 1B provided methodological validation, since hsp70 is already proposed as a marker of cold stress based on data in human transformed embryonic kidney (HEK293T) cells 48.
After 1h treatment with ferric citrate 10 μmol/L, no genes were differentially expressed (Supplementary Table S2). This would be expected given the diurnal peak to trough difference in mean serum iron of 12 μmol/L which circulating cells need to withstand 29.
After 1h treatment with 100 μg/mL cycloheximide to inhibit protein translation as occurs during the integrated stress response 14, 15, 16, 3,819 genes were differentially expressed to p<0.05 including 166 genes meeting genome-wide significance at Benjamini adjusted p<1.5×10-8, and 6 to Benjamini p<10-30 (Supplementary Table S3A). The top five most differentially expressed genes included IL1B and TNF. A volcano plot (Figure 2B) highlighted that the most differentially expressed genes were primary response genes (also referred to as immediate early genes), that are activated within 5-10 mins of a stress 50. Gene ontology (GO) enrichment analyses of all 166 genes where the adjusted p-value was <1.5×10-8 highlighted primary response gene-expected processes related to acute inflammation and mitogen-activated protein kinase (MAPK)/ extracellular signal–regulated kinase (ERK) cascades (Supplementary Figure S2, Supplementary Table S3B).
We considered mechanisms by which these rapid, 1hr changes were occurring. Since cycloheximide inhibits protein translation which is required for NMD, we tested the overlap of differentially expressed genes in the current study with major datasets for NMD-specific inhibition (72hr siRNA inhibition of UPF1 51; knockdown of SMG5, SMG6 or SMG7 52). These studies had been performed in different cell types (human HeLa cells 51 and mouse embryonic stem cells(ESCs) 52, but overlapping genes included the four widely-expressed PBMC genes most differentially expressed after cycloheximide (Supplementary Table S3A). These were for NMD-inhibited ESCs, JUN (PBMC Benjamini-adjusted p=1.1×10-51), ATF3 (PBMC p=1.5 x10-22), and OSM (PBMC p=1.9 x10-26), 51 and for NMD-inhibited HeLa cells, PPP1R15A (PBMC p=1.2 x10-19) 52. The findings were in keeping with a role for NMD in the acute 1hr responses to cycloheximide in PBMCs (though clearly not for responses to 32°C as shown by the intronless HSPA1B).
Conventional and new PBMC isolation methods
Of the 60 publications retrieved April 2022-June 2023 using search terms “PBMC” and “RNASeq”, 27 described isolation methods. As shown in Figure S3A, five referred to buffy coat preparation in detail, with four describing an initial 50:50 dilution of blood with phosphate-buffered saline (PBS) prior to layering over Ficoll and the fifth referring to a PBS-blood mixture for Leucosep tubes. A further 22 referred to methods that protocolise 50:50 dilution of blood with PBS (Ficoll N=20; Histopaque N=1) or PBS/2% FBS (Lymphoprep, N=1) with one also referring to Leucosep preparation where PBS dilution is optimal. All 27 papers referred to buffy coats as PBMCs and referred to methods where one to three PBS wash steps are recommended before resuspension in serum-supplemented media optimised for cells in suspension (RPMI, Roswell Park Memorial Institute media). We concluded that all had performed RNASeq on cells that during isolation and/or wash steps had been removed from their endogenous plasma. To test whether the endogenous plasma resuspension protocol mitigated isolation-associated stress responses compared to conventional protocols, we recruited six new donors for a direct comparison. As endogenous plasma-resuspended PBMCs demonstrated resilience to a 10 µmol/L ferric citrate challenge, we increased the ferric citrate concentration to 40 µmol/L to enhance the likelihood of generating differential gene expression and to explore the limits of plasma protection in our protocol.
Basal induction of stress signalling pathway genes in PBMCs following standard wash/plasma isolation
For six new donors, PBMCs were prepared by two simultaneous methods before stress treatments. All were prepared using plasma resuspensions as previously (Supplementary Figure S3B), and in addition, either by ‘Wash Method 1’ which used an in-house standard Ficoll protocol (donors 4-6), or ‘Wash Method 2’ which used washes as recommended for PBMCs preparation using BD Vacutainer® CPT™ tubes (donors 7-9). In replicate preparations for each method, all replicates were consistent and met a coefficient of variation <10% (CV10) for RNA quality assessed by RNA quality number (RQN).
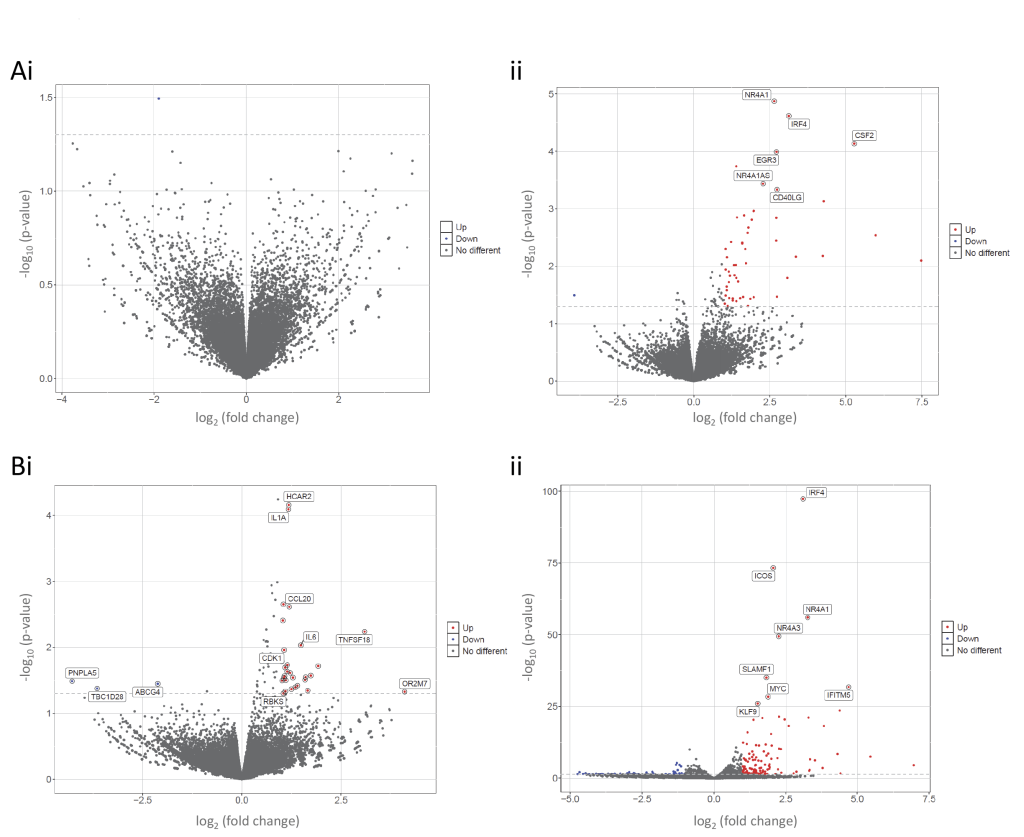
For otherwise untreated cells, compared to plasma-resuspended PBMCs from the same donors, Wash Method 1 (Ficoll preparation) resulted in differential expression of 6,204 genes to unadjusted p<0.05, with 205 genes meeting genome-wide significance at p<1.5×10-8, including 13 to Benjamini p<10-30 (Figure 3A, Supplementary Table S4). These clustered to mitogen activated protein kinase (MAPK), cytokine and other stress signalling pathways (Figure 3B, Supplementary Figure S4).
–
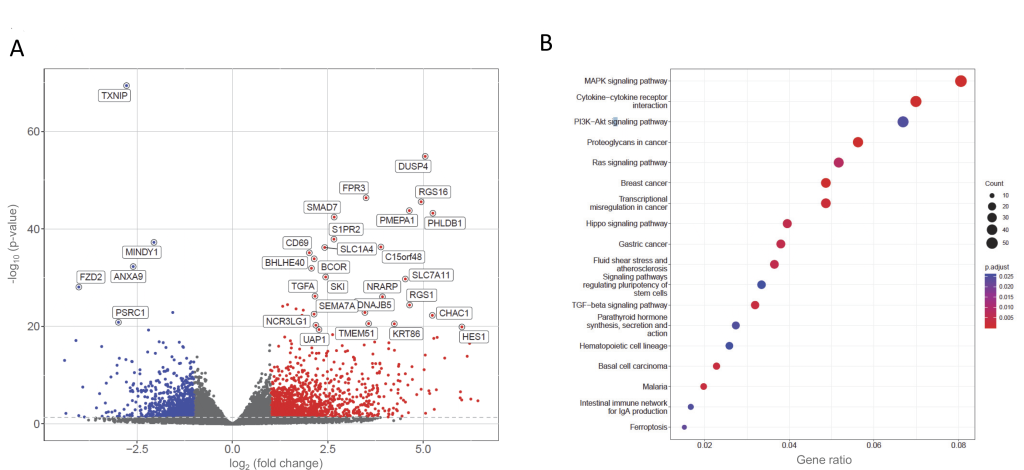 |
FIGURE 3: Basal differences in same-donor human peripheral blood mononuclear cells (PBMCs) prepared by Wash Method 1 compared to plasma resuspension. (A) Volcano plot of the 2,388 genes differentially expressed to p<0.05 in Ficoll-prepared PBMCs from Donors 4-6 that were washed and resuspended in serum-supplemented RPMI media (Wash Method 1), compared to simultaneously-prepared PBMCs from the same donors that were not washed, and were maintained in endogenous plasma throughout preparation, treatment and final centrifugation steps. Thresholds for the volcano plot of genes differentially expressed to p<0.05 were log2(fold change) >1(red) and <1 (blue). (B) KEGG (Kyoto Encyclopaedia of Genes and Genomes) [53–55] pathways identified by the differentially expressed genes. |
To test if the difference was in the washes/media or Ficoll versus CPT™ tube components, we compared untreated PBMCs prepared using BD Vacutainer® CPT™ with washes as in the manufacturer’s protocol (Wash Method 2), to plasma-resuspended PBMCs from CPT™ tubes in three further donors. Wash Method 2 resulted in differential expression of 919 genes meeting genome-wide significance at p<1.5×10-8, including 102 to Benjamini p<10-30, (Figure 4A, Supplementary Table S5A). Again, these clustered to mitogen activated protein kinase (MAPK), cytokine and other stress signalling pathways (Figure 4B, Supplementary Figure S5).
–
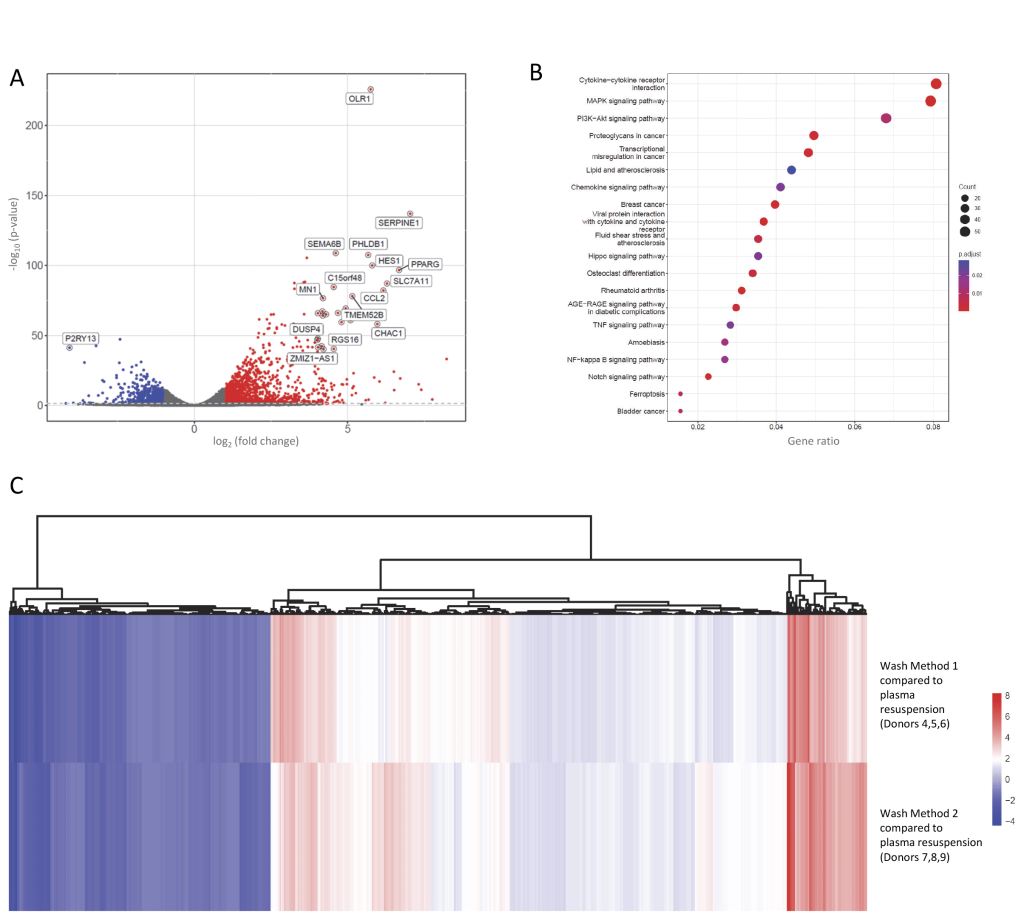 | FIGURE 4: Basal differences in same-donor human peripheral blood mononuclear cells (PBMCs) prepared using CPTTM tubes with and without washes. (A) Volcano plot of the 2,205 genes differentially expressed in PBMCs from Donors 7-9 that were isolated using CPTTM tubes, with washes then resuspension in FCS-supplemented RPMI media, compared to simultaneously-prepared PBMCs from the same donors maintained in endogenous plasma. (B) KEGG pathways [53–55] identified by the differentially expressed genes. (C) Heat map of log2 (fold change) in gene expression for the 1,378 genes that were differentially expressed in PBMCs across both sets of donor trios following washes, compared to plasma resuspended PBMCs from the same donor. As shown in Supplementary Table 5B, the 1,378 genes were those that were differentially expressed for both Wash 1 (coloured symbols in Figure 3A) and Wash 2 (coloured symbols in Figure 4A). Note marked similarities in these genes differentially expressed by the different wash protocols in the different donor trios. |
1,378 genes were differentially expressed to p<0.05 (and log2FoldChange > 1 or < -1) in both the PBMCs from donors 4-6 after Wash 1 and the PBMCs donors 7-9 after Wash 2. The 1,378 genes represented 57.7% of the 2,388 Wash 1 differentially expressed genes, and 62.5% of the 2,205 Wash 2 differentially expressed genes. As shown in Supplementary Table 5B, all log2 fold-changes were in the same direction (Spearman correlation 0.95). Notably, where a gene was strongly downregulated, the gene was always strongly downregulated in both datasets (Figure 4C, deep blue, left). A smaller number of genes were strongly upregulated, where the magnitude of upregulation was more variable (Figure 4C, deep red, right). Overall, the heat maps confirmed the similarity in genes that were differentially expressed after Washes 1 and 2 (Figure 4C), and we concluded it was the washes/resuspension that was generating the differential gene expression profiles rather than the specific method of cell separation.
Pre-activated, washed PBMCs display fewer differentially-expressed gene signatures after cycloheximide, compared to same-donor, plasma-resuspended PBMCs
For Wash Method 1 (Ficoll) in donors 4-6, differential expression patterns after cycloheximide (Figure 5A, Supplementary Table S6) resembled those in PBMCs from donors 1-3 that were plasma-resuspended (Figure 2B). However, in keeping with their basal changes in gene expression similar to an activation profile, fewer clusters were derived compared to the same-donor PBMCs isolated in plasma (Figure 5B). Notably absent were the terms for the MAPK and TNF signalling pathways that had been identified by genes differentially expressed in the same donors in untreated, Ficoll-prepared PBMCs (Figure 3B).
–
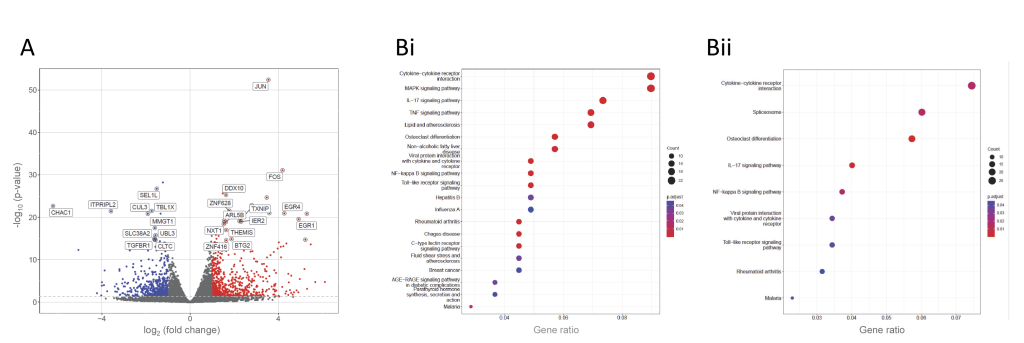 |
FIGURE 5: Differential gene expression in same-donor human peripheral blood mononuclear cells (PBMCs) prepared by wash method 1 after 1h cycloheximide treatment. (A) Wash method 1 (Ficoll-prepared) PBMCs compared to same-donor, plasma-resuspended PBMCs (donors 4-6). (B) KEGG pathways [53–55] identified by genes differentially expressed after 1h cycloheximide 100µg/mL in i ) plasma-resuspended PBMCs and ii) same-donor Ficoll-prepared PBMCs (Wash Method 1). |
Similarly, in PBMCs prepared using Wash Method 2 (BD Vacutainer® CPT™ tubes) from donors 7-9, there was a differential gene expression signature (Figure 6A, Supplementary Table S7) but pathways were enriched to lower significance levels than plasma-resuspended PBMCs from the same donors, with loss of many pathway terms (Figure 6B). We concluded that cycloheximide-specific differential gene expression was being masked by preparative-associated changes.
–
 |
FIGURE 6: Differential gene expression in same-donor human peripheral blood mononuclear cells (PBMCs) prepared by Wash Method 2 after 1h cycloheximide treatment. (A) Wash method 2 (CPTTM)-prepared PBMCs compared to same-donor, plasma-resuspended PBMCs (donors 7-9). Note similarity with subset of genes in Donors 1-3 (Fig. 2B). (B) KEGG pathways [53–55] identified by genes differentially expressed after 1h cycloheximide 100µg/mL in i) plasma-resuspended PBMCs and ii) same-donor Wash Method 2-prepared PBMCs. |
Subset of genes and pathways shared in differential expression profiles after washes or cycloheximide
It was recognised that both washes and cycloheximide treatment of plasma-resuspended PBMCs were generating similar KEGG pathway profiles from differentially expressed genes. To examine in more detail, we reviewed the intersections between the two stresses in the respective donors. As shown in Figure 7 for both donors 4-6 (Figure 7A) and donors 7-9 (Figure 7B), a modest proportion of genes were differentially expressed in both datasets, and these did not always change significantly in the same direction, yet they generated apparently identically significant KEGG term profiles. This was not a methodological error- as shown in Supplementary Table S8, the same KEGG pathways were generated through different genes. We concluded that PBMCs were achieving these core stress responses by modifying changes of different gene subsets.
–
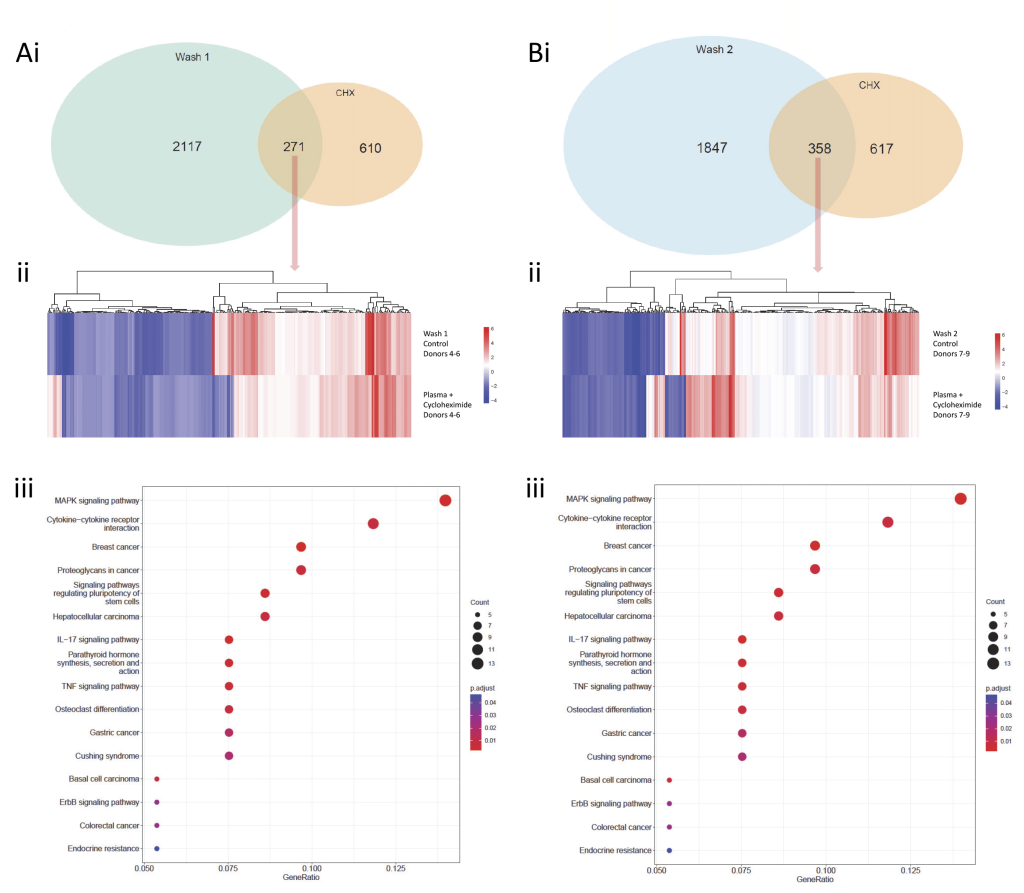 | FIGURE 7: Shared differentially expressed genes in same-donor human peripheral blood mononuclear cells (PBMCs) following ‘treatments’ by either Wash preparation and 1h control treatment, or plasma-resuspension and 1h cycloheximide treatment. (A) Wash method 1 (Ficoll-prepared) PBMCs compared to same-donor, plasma-resuspended PBMCs (donors 4-6). (B) Wash method 2 (CPTTM)-prepared PBMCs compared to same-donor, plasma-resuspended PBMCs (donors 7-9). i) Venn diagrams of genes differentially expressed in each setting, highlighting genes differentially expressed in both settings. ii) Heat maps of log2 (fold change) in gene expression for the 271 genes (Donors 4-6) or 358 genes (Donors 7-9) that were differentially expressed in PBMCs across both treatments. iii) KEGG pathways [53–55] identified by the 271 genes (Donors 4-6) or 358 genes (Donors 7-9). These identical representations resulted from differential expression of different genes for each term as shown in Supplementary Table S8. |
Variable, low-grade responses to 1h 40 μmol/L ferric citrate in same-donor plasma-resuspended PBMCs
PBMCs from donors 4-6 were resilient to 1hr of 40 μmol/L ferric citrate: Only one gene, LINC02967 encoding a long noncoding RNA, was differentially expressed to crude p<0.05 (adjusted p>0.9999) in plasma-resuspended PBMCs (Figure 8Ai). For donors 7-9, three genes (CCL4, HCAR2 and IL1A) exhibited increases to unadjusted p-value <10-4, due to increases in two of the three donors after iron treatment. On re-examining raw data for the 10 µmol/L treatments, two of donors 1-3 had possibly exhibited a similar, less pronounced pattern (Supplementary Table S9). Noting interleukin (IL)1α directly senses DNA damage which was the earliest change identified in primary endothelial cells treated with 10 μmol/L iron for 10 minutes 11, the data could be consistent with iron-induced injury restricted to a minority of donors’ PBMCs when protected by endogenous plasma.
–
Preactivated washed PBMCs display enhanced responses to 1h ferric citrate 40 μmol/L compared to same-donor plasma-resuspended PBMCs
There was a striking difference following 1h ferric citrate 40 μmol/L treatment of same donor PBMCs that had been washed and resuspended in serum-supplemented media. In donors 4-6, only one gene had met unadjusted p<0.05 when plasma-resuspended PBMCs were treated. However, for washed/media-resuspended PBMCs, 83 genes met p<0.05 after 1h ferric citrate 40 μmol/L (Figure 8Aii, Supplementary Table S10) with the top three KEGG terms for cytokine-cytokine receptor interactions, JAK-STAT signaling and T cell receptor signaling (Supplementary Figure S6A). For donors 7-9, 558 genes were differentially expressed to unadjusted p<0.05 (Supplementary Table S11), and 25 met genome-wide significance with p<1.5×10-8 (Figure 8Bi), with very strong overlaps to the most differentially expressed genes in Donors 4-6. Again, top KEGG terms included cytokine-cytokine receptor interactions, JAK-STAT signaling and T cell receptor signaling Supplementary Figure S6B). GO terms 56, 57 generated by clustering differentially expressed genes after 1h ferric citrate 40 μmol/L (Supplementary Figures S7), were consistent with PBMCs additionally triggering adaptive immunity pathways. Overall, data were consistent with a protective role for plasma, and more pronounced, and consistent activation of immune response by PBMCs that had been washed and resuspended in serum-supplemented media compared to endogenous plasma-resuspended PBMCs.
DISCUSSION
We describe a refined system for preparation of human PBMCs within their endogenous plasma and show this provides a window on diverse gene expression profiles and mechanisms. We also show that plasma resuspension limited cellular stress during 1h at 37°C, or under mild hypothermic or ferric citrate stress, compared to conventional PBMC preparative approaches involving washes and resuspension in serum-supplemented media. There were consistent responses across donors to the strong stress from 1h cycloheximide inhibiting protein translation, with NMD implicated as a mechanism, though discrimination of differential gene expression profiles was lessened in washed/media-resuspended cells. More variable responses were observed across donors for experiments with ferric citrate, where in plasma-resuspended PBMCs, there could be an undetectable response to 10 or 40 µmol/L for 1h. Induced or augmented differential gene expression in washed/media-resuspended PBMCs suggested lesser cellular resilience to 10 μmol/L ferric citrate when combined with a second stress.
Limitations of the study were the small number of trio donors for each comparison (though these generated consistent and reproducible results), the application of bulk sequencing across PBMCs rather than limitation to particular subsets or single cells, and that we have not yet formally established mechanisms. However the overriding study strength which we considered important to share promptly in order to maximise the information from other datasets and experiments in design, was attention to minimising acute peri-preparative cellular stress. For datasets already generated, where these exhibit cellular variability 6, 7, 8, 9, the degree to which the preparation-related gene expression profiles (Figure 4C, Supplementary Tables S4, S5) differ between replicates may be a helpful tool to improve normalisation strategies. We extended PBMC examinations beyond effector cell roles in immune and thrombotic processes 3, 4, 5, to a potential test system for human genes where the main tissue of expression is difficult to sample, particularly in studies of patients or genotype-targeted donors 2. In this regard, it was encouraging to note the overlaps with genes highly expressed across five different tissues, and expression of more than half of the genes where Pharmacogenomics is recommended to modify drug dosage in the setting of specific DNA variants.
Another study strength was to model patho-physiologically processes that are broadly relevant, and include a focus on emerging concepts of adaptive and maladaptive responses to cell stressors 29, 46, 47. Ferric citrate 10 μmol/L had no discernible impact in plasma-resuspended PBMCs treated for 1h. At higher concentrations (40 µmol/L), when genes could be differentially expressed by donors’ plasma-resuspended PBMCs (two of donors 7-9), the iron-induced profile was compatible with the earliest signals observed in primary endothelial cells in vitro, namely DNA damage observed after 10 mins after 10 μmol/L iron treatment11. The same 40 µmol/L ferric citrate treatment in the same donors’ PBMCs that had been washed and resuspended in conventional media generated a clear cytokine and innate immunity transcriptome response in all washed/media-resuspended PBMCs. The mild stress to PBMCs during washes and media resuspension that was evident after 1h culture at 37°C modified cell behaviour to a mild stress delivered within the 1h culture period. It is not possible from the current data to opinion whether this was in the direction of greater susceptibility to injury, or a more exuberant and protective cellular response, and further experimental study is recommended.
Cycloheximide treatment data causally linked protein translation inhibition (which occurs transiently as part of the integrated stress response to diverse cellular stressors, 14, 15, 16) to upregulation of primary response genes/immediate early genes 50, and MAPK/ERK cascades. Mechanistically, immediate early genes were known to have low level constitutive transcription, with production of mature, protein-coding transcripts requiring a signal-induced switch 58 mediated by the MAPK pathway proteins via dual phosphorylation of ERK1 and ERK2 59, 60. Our data additionally implicate inhibition of NMD as a focus for future stress studies. Such studies are relevant beyond post-injury responses, as there is growing evidence that these genes and innate immune responses can promote adaptation to environmental stimuli by physiological inflammation in the absence of tissue damage61. In neurological fields, immediate early genes have long been used to visualize neural activations influenced by acute sensory and behavioural stimuli, 62, 63 though there is also evidence of potential for maladaptation with cFOS now implicated in alteration of learning and memory by early-life stress 47.
In conclusion, the findings illuminate several new areas for experimental study of stress responses, and that buffy coat/PBMC isolation in endogenous plasma protects cells from mild stresses, avoiding preactivation signals reported by others 6, 7, 8, 9. Plasma-resuspended PBMCs exhibited marked sensitivity to 1hr inhibition of protein translation, as occurs after cytoplasmic sensing in the integrated stress response; more modest sensitivity to a 1hr 32°C cold stress; and modest or undetectable responses to 1hr of 10 or 40 µmol/L ferric citrate. Isolating PBMCs through conventional washes with media resuspensions altered differential profiles, lessening the ability to detect the full range of responses to cycloheximide, and enhancing cellular responses to 40 μmol/L ferric citrate. For existing datasets, additional normalisation profiles can be proposed based on the degree of basal activation in control cells. Where experimentally feasible, wider use of endogenous plasma isolation and resuspension systems are encouraged.
MATERIALS AND METHODS
This study was approved by the East of Scotland Research Ethics Service (EoSRES: 16/ES/0095), and all participants provided written informed consent.
Donors
All donors reported in the main manuscript were healthy adult volunteers. Each experimental group comprised 3 donors spanning both sexes and a 4-decade age range. All provided written informed consent.
PBMC isolation methods
Literature search
PBMC isolation methods used by other groups were examined following a 29/06/2023 PubMed search using search terms “PBMC” and “RNASeq” (Figure S3A).
PBMC isolation using wash-free, plasma resuspension
Using methods we have optimised in the last ten years, human PBMCs were prepared using BD Vacutainer® CPT™ tubes (Bunzl Healthcare, Coalville, UK) which contain buffered sodium heparin anticoagulant, a liquid density medium and an inert gel barrier that on centrifugation separates erythrocytes and granulocytes from mononuclear cells and platelets (Figure S3B). Two 8 mL CPT™ tubes of blood were taken per individual donor, inverted immediately 8-10 times, and centrifuged within 2hrs of collection at room temperature for 30min at 1600 g relative centrifugal force (RCF). The plasma and buffy coat above the gel were removed with a pipette and pooled in a single 50ml tube per donor. Contents were immediately mixed and distributed in equal volumes (~5 ml) to experimental tubes.
Wash Method 1: Ficoll Preparation
A standard in-house Ficoll protocol for PBMC preparation was used. 15 mL of Ficoll-Plaque Plus solution (VWR, Lutterworth, UK) was aliquoted into two sterile 50 mL tubes. Blood from a single donor (three 8 mL sodium citrate tubes) was pooled and diluted with equal volume PBS (Sigma) within 1h of blood collection: 25 mL of the diluted blood was layered on top of the Ficoll in each of the two tubes that were centrifuged at 800 g (RCF) for 20 mins at room temperature with low brake. The buffy coat above the Ficoll was collected using a sterile Pasture pipette, transferred to two new sterile 50 mL tubes, diluted 1:1 with PBS (Sigma) and centrifuged at 200g (RCF) for 10 mins at room temperature. Two PBS washes were undertaken in keeping with published and local protocols. After removal of the final wash/supernatant, PBMCs were resuspended in 10 mL of RPMI + 2.5% fetal calf serum (FCS), and distributed in equal volumes (~5 mL) to experimental tubes.
Wash Method 2
CPT™ tubes: This method was used to specifically examine the effect of washes and resuspension in RPMI/FCS compared to endogenous plasma. As for the plasma resuspension method, two 8 mL CPT™ tubes of blood were processed to generate the buffy coat. Combining the manufacturer’s recommended washes/media resuspension to mirror the Ficoll protocol, plasma and buffy coat/platelets above the gel were then removed with a pipette, pooled in a single 50 mL tube per donor, diluted 1:1 with sterile PBS (Sigma), then centrifuged for 10 mins at 200 g RCF at room temperature. Two further PBS washes were undertaken in keeping with published and local protocols for Ficoll preparations (see above). After removal of the final wash/supernatant, PBMCs were resuspended in 10mls of RPMI + 2.5% FCS, and distributed in equal volumes (~5 mL) to experimental tubes.
Stress treatments
A fresh stock concentration of 10 mM ferric citrate was generated on the day of each experiment by diluting in molecular biology water and filter-sterilising as described2, 11, 28. Prior to experimental days, biotechnology performance-certified cycloheximide (Sigma C1988) that inhibits protein translation19, 20, was diluted in DNase/RNase free molecular biology water to a stock concentration of 10 mg/mL (100X), filter-sterilised, and frozen in separate aliquots that were defrosted on the morning of the sampling and experiment. On the day of the experiment, a tissue culture water bath was set to 32°C.
For the first 1hr experimental treatments (donors 1-3), after their 10 mins 37°C prewarm, the buffy coat/plasma resuspensions were treated either with 1/1000 Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS) (control, hypothermia); 1/1000 dilution of the freshly prepared ferric citrate to a final concentration of 10 μmol/L, or 1/100 dilution of the defrosted cycloheximide stock solution to a final concentration of 100 μg/mL. Control, iron-treated and cycloheximide-treated experimental tubes were incubated in a 37°C, 5% CO2 incubator, with the hypothermia tube immersed in the 32°C water bath for 1h2. For the comparator experimental treatments (donors 4-9), the 37°C prewarm was extended to 15 mins, the buffy coat/plasma resuspensions were treated either with control, ferric citrate at an increased concentration of 40µmol/L to enhance the likelihood of differential gene expression, or cycloheximide as previously. All experimental tubes were incubated in a 37°C, 5% CO2 incubator for 1h and immediately after treatments, experimental tubes were centrifuged at room temperature for 15 mins at 520g RCF. The unwashed cell pellet was lysed in TRI reagent (Cambridge Bioscience, Cambridge, UK), distributed to two technical replicate microcentrifuge tubes, and stored immediately below -70°C.
RNA sequencing and quality control
RNA extraction and quality control was performed by Genewiz (Leipzig, Germany). The RNA quality number (RQN) was determined from the entire electrophoretic trace for a given total RNA sample including the 18S to 28S ribosomal peak ratio, the separation between these peaks, and the presence or absence of degradation products in the fast region. Potential RQN values range from 10 to 1, where 10 indicates the highest possible RNA quality and 1 indicates strongly degraded RNA.
For the RNASeq data described in this manuscript, samples were polyA-selected for polyadenylated RNA enrichment, fragmented and random primed for first and second strand cDNA synthesis, end repair, 5’ phosphorylation, dA-tailing, adaptor ligation, PCR enrichment and Illumina HiSeq sequencing using paired-end 150 bp reads (Genewiz, Leipzig, Germany). Sequenced reads were trimmed using Trimmomatic v.0.36 64, aligned to Homo sapiens GRCh38 65 using STAR aligner v2.5.2b, and unique gene reads that fell within exon regions counted using Subread package v1.5.2 (Genewiz, Leipzig, Germany). The coefficient of variation (CV) 66 for each transcript for each replicate pair was calculated as the standard deviation/mean. Technical replicates where the CV was <10% were designated as meeting CV10.
RNASeq analyses
For targeted expression analyses, untreated PBMC mean transcript reads were examined for the top 100 expressed transcripts in five major tissues as downloaded from the Genotype Tissue Expression Project,41 and a test dataset of the 17 genes with actionable guidance in clinical practice, generated from the Pharmacogenetics Knowledgebase42, the Dutch Pharmacogenomic Working Group 43, and the Clinical Pharmacogenetics Implementation Consortium 44.
Differential gene expression including DeSeq2 v1.42.1 normalisations67 were performed using customised scripts in R v4.3.0 68. Across each set of 3 volunteer donors, the mean base values were calculated across the 2 replicate libraries per donor for all conditions except one where inadequate RNA yields prevented generation of one library. Log2 fold changes between comparator experiments (preparation method or treatment), and Benjamini-adjusted p-values were calculated. For Volcano plots, KEGG and GO enrichment analyses in Figures 3-8, differential gene expression was considered by log2FoldChange > 1 or < -1 and p<0.05 as specified. Volcano plots of log2FoldChange against -log10 of the p value or adjusted p value were used to visualise results by ggplot2 v3.5.1 69. GO enrichment analysis was performed in R using clusterProfiler v4.10.1 70.
Data availablility statement
Non-sensitive data underlying this article are uploaded to Zenodo as the Genewiz-provided read counts in the RNASeq libraries (https://zenodo.org/records/14215620 ). They can be used under the Creative Commons Attribution licence.
SUPPLEMENTAL INFORMATION
![]() Download Supplemental Information
Download Supplemental Information
ACKNOWLEDGMENTS
Funding support was from the NIHR Imperial Biomedical Re- search Centre and the D’Almeida Charitable Trust. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. Data for the current study were downloaded on 01 November 2021. We thank our academic and public partners in the NIHR Imperial BRC Social, Genetic and Environmental Determinants of Health Theme, and also thank the donors for their willing participation in these studies.
COPYRIGHT
© 2024

Endogenous plasma resuspension of peripheral blood mononuclear cells prevents preparative-associated stress that modifies polyA-enriched RNA responses to subsequent acute stressors by Li et al. is licensed under a Creative Commons Attribution 4.0 International License.