Back to article: Spermidine supplementation in rare translation-associated disorders
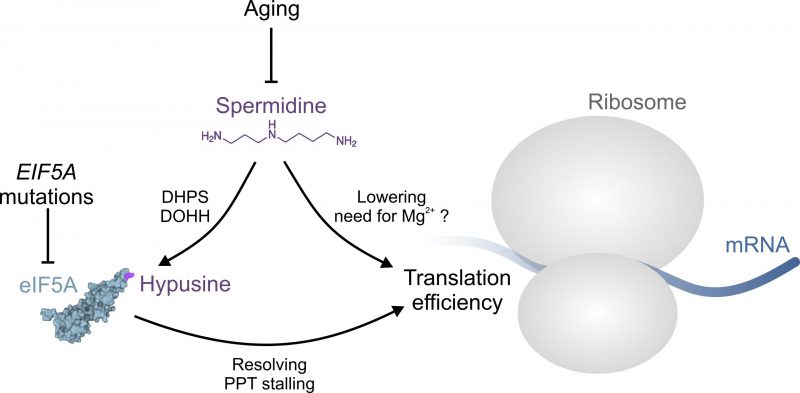
FIGURE 1: The role of spermidine in protein translation. Aging or EIF5A mutations can interfere with normal protein translation through insufficient eIF5A hypusination and/or function, respectively. Aging is accompanied by a decrease of available spermidine, which is required for the hypusination of eIF5A through deoxyhypusine synthase (DHPS) and deoxyhypusine hydroxylase (DOHH). Hypusinated eIF5A is able to resolve ribosomal stalling at poly-proline tract (PPT) motifs. Spermidine may promote translation hrough eIF5A-independent mechanisms, e.g. by lowering the need for Mg2+ ions. See text for details. The structure of eIF5A was generated based on the RCSB PDB (rcsb.org) entry 3ER0 representing the crystal structure of yeast eIF5A [19].
19. Sanches M, Dias CAO, Aponi LH, Valentini SR, and Guimaraes, B (2008). Crystal structure of the full length eIF5A from Saccharomyces cerevisiae. 10.2210/pdb3ER0/pdb