Back to article: TRIM16 employs NRF2, ubiquitin system and aggrephagy for safe disposal of stress-induced misfolded proteins
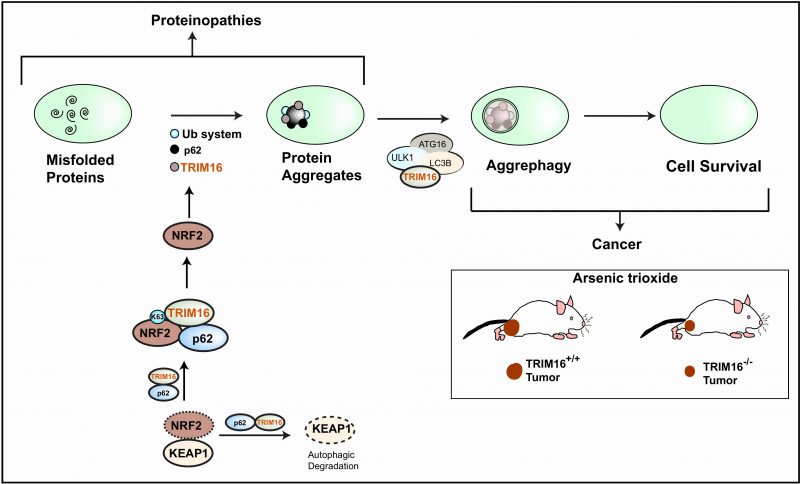
FIGURE 1: TRIM16 maintains protein homeostasis by regulating NRF2 and aggrephagy. The TRIM16 (tripartite motif-containing protein 16) interacts with NRF2 (nuclear factor erythroid 2 like 2) and p62/SQSTM1. TRIM16 displaces KEAP1 (Kelch-like ECH-associated protein 1) and enhances K63-linked ubiquitination of NRF2 to stabilize its expression. The activated NRF2 induces expression of p62, TRIM16 and ubiquitin pathway genes. All the three play an important role in the conversion of stress-induced misfolded proteins into protein aggregates. TRIM16 is present over the protein aggregates and acts as a scaffold protein to bring the autophagy adaptor proteinp62, the autophagy initiation protein ULK1 (Unc-51 like autophagy activating kinase 1), the autophagy elongation protein LC3B (microtubule associated protein 1 light chain 3 beta), and ATG16L1 (Autophagy related 16 like 1) over the protein aggregates for aggrephagy. This mechanism of turnover of misfolded proteins could be protective in proteinopathies. However, cancer cells exploit this mechanism for their survival. Therefore, we found that in a xenograft mouse model, TRIM16 knockout (TRIM16-/-) tumors were regressed after exposure to oxidative stress (Arsenic trioxide).