Back to article: Alzheimer’s disease: amyloid-based pathogenesis and potential therapies
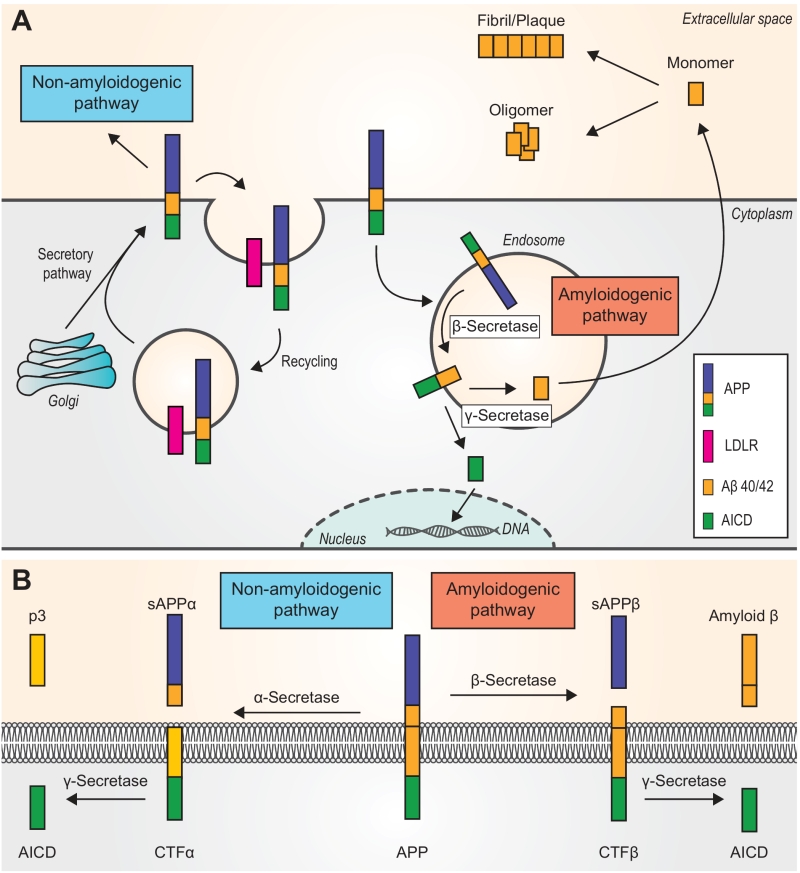
FIGURE 1: Two pathways of amyloid β (Aβ) peptides generation. Amyloid precursor protein (APP) is a type I integral membrane protein that is cleaved at different sites to yield various products. (A) An overview of the two pathways. APP is translocated from the cytoplasm into the endoplasmic reticulum where it enters the secretory pathway and then is transported to the neuronal plasma membrane. The majority of APP is processed via the non-amyloidogenic pathway (see panel B). APP can be recycled in endosomes by binding to LDLRs (low-density lipoprotein receptors). The right side of the figure shows the amyloidogenic pathway. Unlike the non-amyloidogenic pathway, which is carried out at the plasma membrane, the amyloidogenic pathway mainly occurs in endosomes. Ultimately, AICD is transferred to the nucleus, where it functions as a transcriptional factor, whereas the Aβ40/42 monomer is removed to the extracellular space. Monomers aggregate either into oligomers or fibrils/plaques. (B) Non-amyloidogenic (left) or amyloidogenic (right) pathway of APP processing. In the non-amyloidogenic pathway, APP is first cleaved by α-secretase, releasing a soluble ectodomain of APP called sAPPα and a membrane-tethered intracellular C-terminal fragment (CTFα). Then, the C-terminal fragment is further cleaved by γ-secretase to produce a 3-kDa peptide (p3) and an APP intracellular domain (AICD). In the amyloidogenic pathway, the products of β-secretase are a soluble ectodomain of APP (sAPPβ) and a C-terminal fragment β (CTFβ). The second step releases Amyloid b and AICD. APP, amyloid precursor protein. CTF alpha/beta, C-terminal fragment alpha/beta. sAPP alpha/beta, soluble ectodomain of APP. p3, 3-kD peptide. AICD, APP intracellular domain.