Back to article: Salmonella Typhimurium infection: Type I Interferons integrate cellular networks to disintegrate macrophages
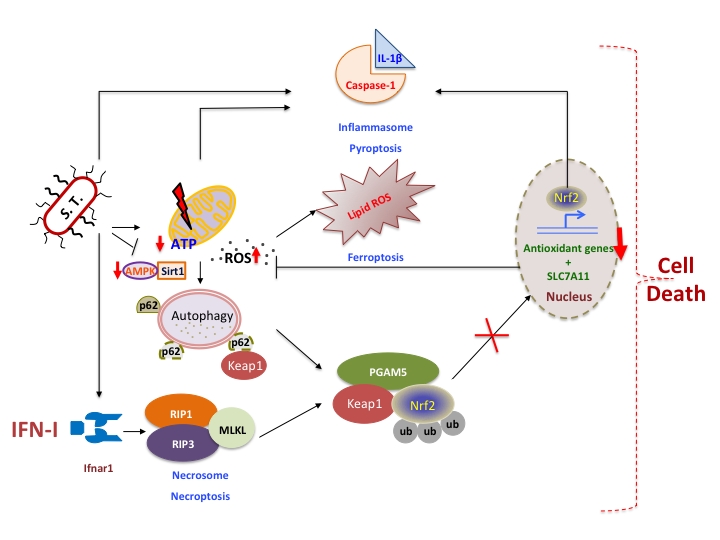
Figure 1: IFN-I integrates cellular networks to induce macrophage-death upon S. Typhimurium infection. S. Typhimurium (S.T) infection in macrophages results in mitochondrial damage hence reduction in ATP and mitochondrial ROS accumulation. This is a trigger for autophagy, however S.T abrogates AMPK and Sirt1 resulting in the inhibition of autophagy and p62 accumulation, which could sequester Keap1 enabling nuclear translocation of Nrf2 and transcription of cytoprotective anti-oxidative genes. However, type I interferons (IFN-I) induced by S.T. activates RIP kinases-mediated necrosome that subsequently activates PGAM5. PGAM5 sequesters Nrf2 in the cytoplasm and enables proteosomal degradation mediated by Keap1. Nrf2 is also known to regulate IL-1β expression, which is correlated with inflammasome activation and pyroptosis. Another cell death pathway termed as ferroptosis is also dependent on lipid based ROS regulated by SLC7A11 which is under the transcriptional control of Nrf2. Thus IFN-I integrates various cross-regulatory cellular networks, which eventually leads to the death of S. Typhimurium-infected macrophages.